PhasED-Seq ctDNA detection enables accurate and predictive treatment response monitoring and clinical prognosis in patients with B-cell lymphoma.
AURORA, Colo., Nov. 29, 2022 /PRNewswire/ -- Foresight Diagnostics, a leader in minimal residual disease (MRD) detection technology, announced today that three studies utilizing their patented PhasED-Seq technology will be presented at the 64th American Society of Hematology Annual Meeting and Exposition (ASH 2022) taking place December 10-13, 2022, at the Ernest N. Morial Convention Center in New Orleans, LA.
The Foresight Diagnostics MRD platform is based on the sequencing of phased variants (PVs) for ultrasensitive and highly specific tumor-derived cell free DNA (ctDNA) detection from solid tumors and B-cell malignancies. PhasED-Seq enhances specificity by requiring the concordant detection of two or more distinct mutations within a single DNA molecule. This enables PhasED-Seq to more accurately distinguish ctDNA from healthy cell free DNA. PhasED-Seq detects ctDNA at levels below one part-per-million (<.0001%), which enables the detection of cancer relapse up to 200 days earlier than other commercial ctDNA detection methods*. The PhasED-Seq platform has been validated in hundreds of real-world patient samples.
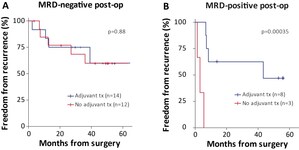
Mark Roschewski, MD, of the National Cancer Institute, part of the National Institutes of Health, will present data, generated in collaboration with MorphoSys AG, demonstrating that PhasED-Seq's sensitivity exceeded that of PET/CT scans and was highly prognostic for outcomes when used to measure MRD at the end of treatment in five clinical trials of patients with DLBCL.
David M. Kurtz, MD PhD, of Stanford University School of Medicine, has authored a poster presenting data, generated in collaboration with MorphoSys AG, on the use of PhasED-Seq to accurately track therapeutic response and predict clinical outcomes of first line therapy combinations in de novo DLBCL.
Dr. Kurtz will also give an oral presentation of data, generated in collaboration with Tessa Therapeutics, from the CHARIOT phase 2 clinical trial that supports PhasED-Seq ctDNA detection as a viable biomarker to assess risk, monitor response, and predict outcomes in patients with recurrent classic Hodgkin Lymphoma treated with CD30.CAR-T therapy.
"We are very pleased to have been selected by the ASH Program Committee to share these impactful clinical studies utilizing Foresight's PhasED-Seq MRD technology for guiding lymphoma treatments and furthering clinical trials," said Jake Chabon, PhD, CEO and co-founder of Foresight Diagnostics. "Our goal is to provide industry leading MRD sensitivity that can provide actionable information to physicians and pharmaceutical companies to enhance a personalized approach to cancer treatment. We are very grateful to our partners at MorphoSys AG and Tessa Therapeutics for including our PhasED-Seq technology in their clinical trials and demonstrating its superior MRD sensitivity and utility for predicting clinical outcomes. We are also grateful to Dr. Roschewski, Dr. Kurtz, and their teams for presenting this important work at the 2022 ASH Annual Meeting."
Abstract Number: 322
Abstract Title: MRD-Negativity as a Potential Surrogate Endpoint after Frontline DLBCL Therapy: Pooled Analysis of Trials & Implications for Clinical Trial Design
Presenting Author: Mark Roschewski, MD, Lymphoid Malignancies Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD
Session Date: Saturday, Dec. 10, 2022, at 4:45 PM CT; session 627, Theater AB
Abstract Summary: Studies were conducted to compare the predictive value of ctDNA MRD detection to radiographic response and ultimate outcomes including progression free survival (PFS) in patients with DLBCL. Cell-free DNA was profiled using Foresight Diagnostics' PhasED-Seq to identify tumor-associated phased variants (PVs) and these PVs were used to monitor MRD at various interim timepoints and at end of treatment (EOT) in 5 prospective studies including investigational combinations of CHOP or EP[O]CH with acalabrutinib, lenalidomide, obinutuzumab, polatuzumab, and tafasitamab. Results show that detection of ctDNA MRD at EOT using PhasED-Seq is more sensitive than PET/CT and highly prognostic for outcomes in DLBCL. Studies suggest that PhasED-Seq's ultrasensitive 1ppm limit of detection may serve as a surrogate clinical endpoint at EOT and should be routinely used in the analysis of MRD in clinical trials for DLBCL.
Abstract Number: 984
Abstract Title: Ultrasensitive Circulating Tumor DNA (ctDNA) Dynamics after Autologous CD30.CAR-T Cell Therapy for Relapsed or Refractory (r/r) Classical Hodgkin Lymphoma (CHARIOT Trial)
Presenting Author: David M. Kurtz, MD Ph.D., Division of Oncology, Stanford University School of Medicine, Stanford, CA
Session Date: Monday, Dec. 12, 2022, 5:45 PM CT; session 704, Hall E
Abstract Summary: The Phase 2 multicenter study, CHARIOT, is investigating the use of CD30.CAR-T cells in cHL patients with progression following 3 lines of therapy. Foresight Diagnostics' PhasED-Seq MRD assay was used to measure ctDNA levels as a possible biomarker at multiple timepoints, including at baseline (pre-treatment), at day 42 post-CD30.CAR-T infusion (D42), and upon progressive disease (PD). Data showed that ctDNA responses correlated with radiographic responses, suggesting that pre-treatment ctDNA levels could be predictive of patient response to CAR-T therapy. Researchers also determined that PhasED-Seq ctDNA analysis is a viable biomarker to assess risk, monitor responses, and predict outcomes in patients with r/r cHL treated with CD30.CAR-T therapy.
Abstract Number: 1519
Abstract Title: Ultrasensitive MRD Profiling Predicts Outcomes in DLBCL after Frontline Therapy with tafasitamab in Combination with lenalidomide and R-CHOP
Presenting Author: David M. Kurtz, MD PhD, Division of Oncology, Stanford University School of Medicine, Stanford, CA
Session Date: Saturday, Dec. 10, 2022, 5:30 PM-7:30 PM CT; session 621, Hall D
Abstract Summary: Studies were conducted to determine if ultrasensitive detection of ctDNA MRD levels could accurately track response and predict curative outcomes following experimental first line (1L) therapy. Serial blood specimens were evaluated from patients enrolled in a randomized Phase Ib study in de novo DLBCL treated with tafasitamab in combination with lenalidomide & R-CHOP (T/LR-CHOP). Cell-free DNA was profiled using Foresight Diagnostics' PhasED-Seq assay, prior to treatment, to genotype each patient's tumor for phased variants (PVs). PV detection was used to monitor MRD in blood specimens collected following 1 cycle, 3 cycles, 6 cycles/end-of-treatment (EOT), and 6-months post-treatment. MRD positivity at each timepoint was associated with likelihood of future progression or death. The results showed that the absence of ctDNA MRD as measured by PhasED-Seq, strongly correlated with durable responses to T/LR-CHOP in DLBCL.
Foresight Diagnostics is a privately held cancer diagnostics company and CLIA-registered laboratory. The company has developed a novel liquid biopsy testing platform for the measurement of minimal residual disease (MRD) that is significantly more sensitive (with a detection limit below 0.0001% or one part-per-million) than existing tests. The improved sensitivity of the Foresight MRD assays can provide actionable information to physicians and biopharmaceutical companies to enable more personalized treatment approaches for patients with solid tumors and B-cell malignancies. For more information, please visit foresight-dx.com and follow us on Twitter and LinkedIn.
The Foresight MRD platform is based on the Phased variant Enrichment and Detection by Sequencing (PhasED-Seq) technology. PhasED-Seq lowers the error profile of mutation detection in sequencing data by requiring the concordant detection of two separate non-reference events in an individual DNA molecule. By detecting more than one mutation, PhasED-Seq can more accurately distinguish tumor-derived cell free DNA (i.e., ctDNA) from healthy cell free DNA - enabling detection of ctDNA at levels below one part-per-million (<.0001%). PhasED-Seq has been exclusively validated in thousands of patient samples.
Contact Foresight
Phone: 720-443-3658
Email: [email protected]
SOURCE Foresight Diagnostics

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In



Share this article