FDA Clears Prelivia, a Medical Device that Protects Patients from Pressure Injuries at Hospitals and Care Homes
VANCOUVER, BC, Sept. 1, 2021 /PRNewswire/ -- The U.S. Food and Drug Administration has provided 510 (k) Clearance for Prelivia, a neurostimulation device developed by Rehabtronics that promotes healthy blood circulation and maintains healthy tissue in people who are bedridden or chair bound. Annually, more than 2.5 million people in the United States suffer from pressure injuries, also known as bed sores, and up to 60,000 people die. Prelivia is the first innovation in 70 years aimed at protecting patients from pressure injuries.
"Every year, more people die from pressure injuries than from automobile accidents in the USA alone. The potential promise of Prelivia to maximize ulcer-free, hospital-free and activity-rich days is enormously exciting," says Dr. David Armstrong, a professor of surgery and an expert in wound care based in Southern California.
Current alternatives used in hospitals and long-term care centers include patient turning, sensors and specialty beds and mattresses, which provide temporary relief.
"Prelivia offers a much-needed alternative to patients at risk for bed sores and their care givers who are required to turn patients every two hours to prevent these injuries," said Dr. Rahul Samant, CEO of Rehabtronics, which developed Prelivia. "Due to COVID-19, even more patients are suffering from unavoidable pressure injuries and if it weren't for the herculean efforts of nurses, the incidence of bedsores would be substantially higher than they are today. Prelivia gives nurses a new tool to help protect their patients from pressure injuries."
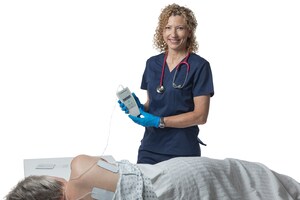
Prelivia uses a patented neurostimulation technology that activates local blood circulation. Studies show that Prelivia's unique technology increases tissue oxygenation by 28% and decreases 80% of pressure induced tissue damage. A caregiver applies electrodes to a patient's skin in the area that is at-risk for developing a pressure injury. Once activated, they stimulate muscle contraction every 10 minutes, which maintains healthy blood flow. Prelivia is painless and can be used continuously.
"With FDA clearance, we can now bring Prelivia into hospital ICUs and care homes to improve patient care and reduce the costs associated with treating pressure injuries," Samant adds. "We are in discussions with leading healthcare facilities to implement Prelivia. We look forward to working with physicians and nurses to transform pressure injury care programs and alleviate unnecessary suffering."
First tested in animal studies, Prelivia's IES technology has been proven to reduce injuries. IES was also recently tested on 68 patients at a healthcare facility in Alberta, Canada– all of whom were considered at-risk of developing a pressure ulcer/bed sore. No pressure injuries were observed while Prelivia was applied over a four-week period.
ABOUT REHABTRONICS
Rehabtronics develops medical devices that restore function and improve the lives of people who are paralyzed or immobile. Founded in 2003 as a spinoff from the Neuroscience Institute of the University of Alberta, Rehabtronics is dedicated to bringing neuroscience discoveries into clinical practice. The company's newest product, Prelivia, is designed to alleviate pressure injuries, one of the deadliest hospital-acquired injuries. Its rehabilitation devices are used globally to help people recover movement after central or peripheral nervous system injury or disease. www.rehabtronics.com.
Media Contact:
Neena Rahemtulla
604-345-1646
[email protected]
SOURCE Rehabtronics

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article