FDA approves new streamlined workflow option for Roche's cobas HPV Test
New process allows labs to use same Pap test vial instead of transferring sample to new vial, thus simplifying workflow
INDIANAPOLIS, June 26, 2013 /PRNewswire/ -- Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that the U.S. Food and Drug Administration (FDA) has approved a new, more efficient workflow process for the cobas HPV Test, allowing sample processing from the primary sample collection vial after it has been used for cytology (Pap) testing. This development allows labs to load the same vial used for a ThinPrep Pap test directly onto Roche's cobas 4800 System for high-risk HPV (Human Papillomavirus) and individual HPV 16 and 18 genotype testing, which is used to help identify women at risk for cervical cancer.
"With new clinical guidelines for cervical cancer screening issued last year, the demand for HPV co-testing has the potential to increase significantly," said Alan Wright, MD, MPH, chief medical officer for Roche Diagnostics. "Giving labs the option to use the same primary vial from a Pap test for HPV testing lets them eliminate a complete step in their workflow process and lower the risk of errors in sample handling, helping to ensure that patients receive accurate test results."
The new workflow option utilizes a special primary vial rack for the fully automated cobas 4800 System. The new process eliminates the need for lab technologists to pipette samples from the primary vials used for liquid-based cytology into a separate tube. Instead, they can load the same vial onto the cobas 4800 System directly after cytology processing. The streamlined workflow can help labs reduce costs, improve turnaround time and free staff to spend time on other critical tasks.
The cobas HPV Test, approved by the FDA in 2011, is the first HPV test to receive FDA approval for loading a Pap sample vial directly onto an automated system and for the use of primary vial samples after cytology processing on either the ThinPrep 3000 (T3000) system or the ThinPrep 2000 (T2000) system.
About the cobas HPV Test
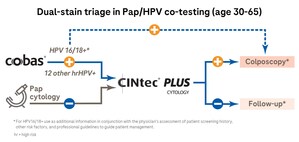
Clinically validated by the landmark ATHENA trial, the cobas HPV Test is the only FDA-approved HPV assay that provides specific genotyping information for HPV 16 and 18, the highest-risk types, while simultaneously reporting the 12 other high-risk HPV types as a pooled result, all in one run, from one patient sample. The test is approved to screen patients age 21 and older with abnormal Pap test results and to co-test with Pap in women ages 30 to 65 to assess the presence or absence of high-risk HPV genotypes.
The test is performed on the cobas 4800 System, which offers true walk-away automation of nucleic acid purification, PCR (polymerase chain reaction) set-up and real-time PCR amplification and detection to help laboratories achieve maximum efficiency. The system also runs the cobas CT/NG Test (chlamydia/gonorrhea), the cobas BRAF V600 Mutation Test and the cobas EGFR Mutation Test.
About Human Papillomavirus and Cervical Cancer
Persistent infection with Human Papillomavirus is the principal cause of cervical cancer in women, with HPV implicated in greater than 99 percent of cervical cancers worldwide. According to the National Cancer Institute, there are 12,200 new cases of cervical cancer in the United States annually and 4,210 deaths due to the disease. The World Health Organization estimates there are 470,000 new cases of cervical cancer annually.
About Roche
Headquartered in Basel, Switzerland, Roche is a leader in research-focused healthcare with combined strengths in pharmaceuticals and diagnostics. Roche is the world's largest biotech company, with truly differentiated medicines in oncology, infectious diseases, inflammation, metabolism and neuroscience. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Roche's personalized healthcare strategy aims at providing medicines and diagnostic tools that enable tangible improvements in the health, quality of life and survival of patients. In 2012 Roche had over 82,000 employees worldwide and invested over 8 billion Swiss francs in R&D. The Group posted sales of 45.5 billion Swiss francs. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com or www.roche-diagnostics.us.
COBAS is a trademark of Roche. ThinPrep is a trademark of Hologic. All trademarks mentioned in this release are protected by law.
For further information, please contact:
| Todd Siesky |
| Director, Corporate Communications Roche Diagnostics Corporation Indianapolis, Indiana USA |
| (317) 521-3966 |
|
|
SOURCE Roche Diagnostics
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article