FDA Approves Abbott's "Low Dose," Recharge-Free Spinal Cord Stimulation System with up to Ten Year Battery Life* for People Living with Chronic Pain
- Proclaim™ XR neurostimulation system uses Abbott's proprietary, low energy BurstDR™ therapy coupled with the BoldXR™ low dosing protocol for safe, effective pain relief and a battery that lasts up to 10 Years*
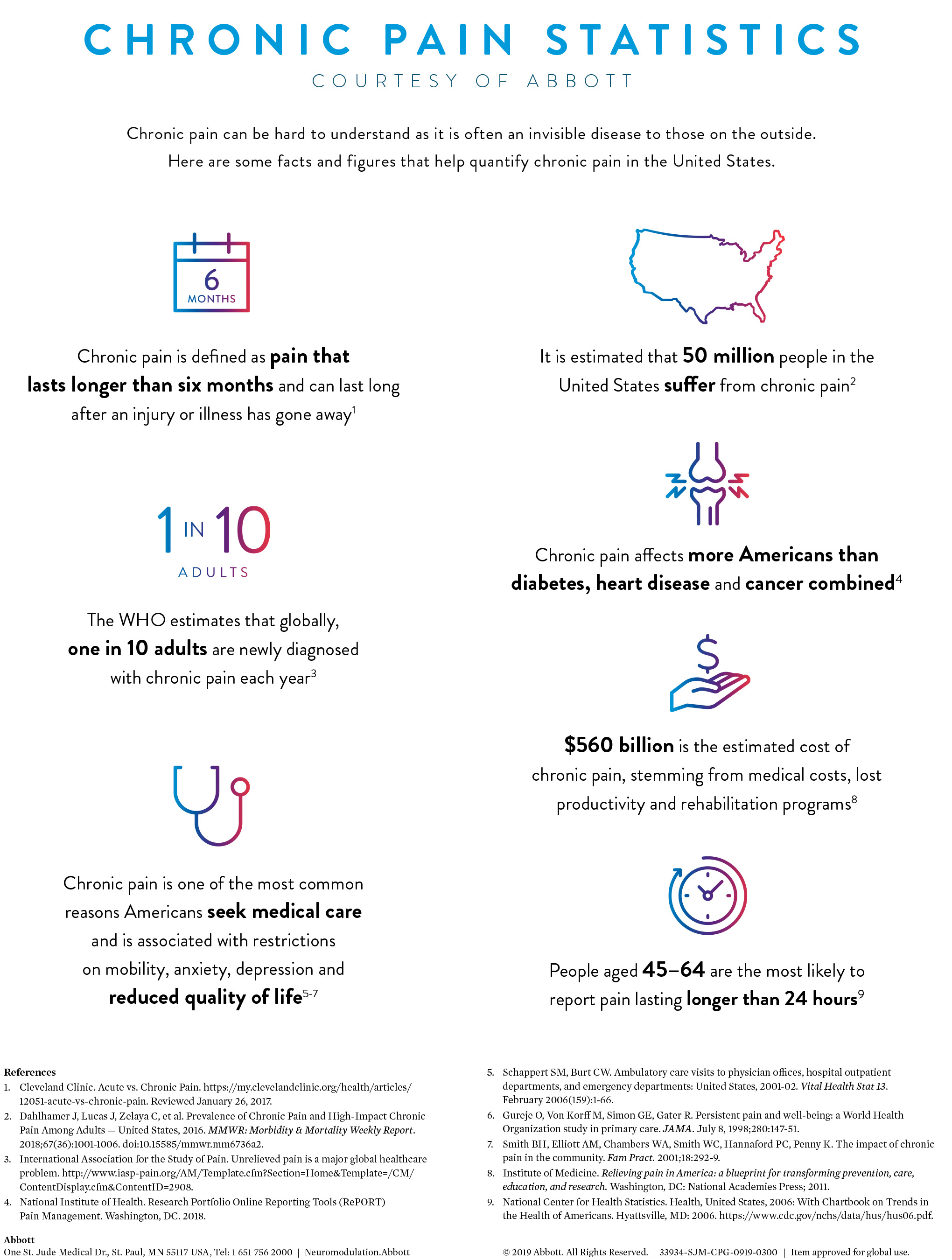
- More than 50 million people in the United States are affected by chronic pain
- Abbott's low-dose and low-energy pain management technology is designed to provide pain relief and improve system longevity, offering patients a life-altering option for pain relief without the hassles of recharging
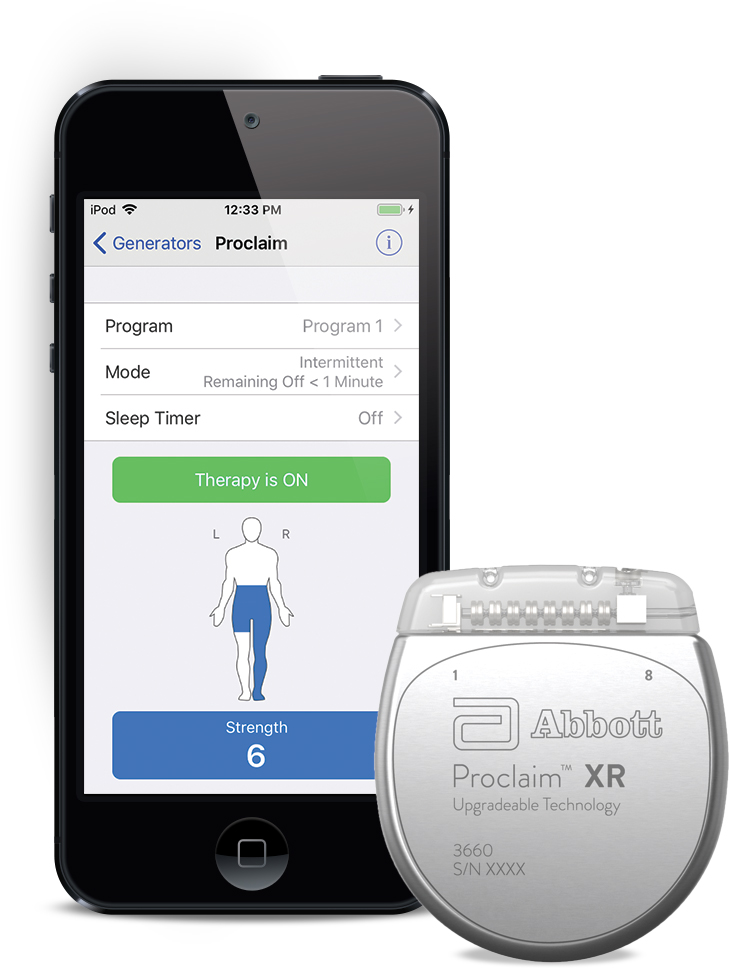
ABBOTT PARK, Ill., Sept. 26, 2019 /PRNewswire/ -- Abbott (NYSE: ABT) today announced the U.S. Food and Drug Administration (FDA) has approved the company's Proclaim XR™ recharge-free neurostimulation system for people living with chronic pain. The Proclaim XR platform offers a low dose of Abbott's proprietary BurstDR™ stimulation waveform, which was created based on scientific insights from doctors and research to mimic natural patterns found in the brain. It works by using low doses of mild electrical pulses to change pain signals as they travel from the spinal cord to the brain. The delivery of lower doses of spinal cord stimulation (SCS) helps extend the system's battery life, allowing people to experience pain relief, without the hassle of recharging, for up to 10 years*.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8608451-abbott-proclaim-xr-spinal-cord-stimulation-system/
Abbott's low-energy stimulation is based on finding the lowest effective dose as determined by the treating clinician for select patients. It delivers BurstDR stimulation intermittently, at low energy levels, while allowing people to experience the same level of superior pain relief. Proclaim XR also uses familiar Apple mobile digital devices and Bluetooth® wireless technology to help discreetly manage pain and fit seamlessly into a patient's lifestyle.
The system was developed based on positive results from Abbott's BurstDR micrOdosing stimuLation in De-novo patients (BOLD) study, which showed 100% of 24 enrolled patients on a low-energy BurstDR dosing program experienced pain relief with less than six hours of battery use per day, while approximately 50% of those patients achieved pain relief with the lowest effective dose (less than two hours of battery use per day). Even at the highest settings, the systems were still in use only 25% of the time. Data from this trial was presented at multiple society meetings including the North American Neuromodulation Society (NANS) and the International Neuromodulation Society (INS) earlier this year.
"For the 50 million people living with chronic pain in the United States this is a new and exciting treatment that is supported with evidence validated by the BOLD study, an established protocol for titrated intermittent dosing to give patients individualized pain relief while using therapy for 6 hours or less per day," said Timothy Deer, M.D., DABPM, president and chief executive officer of The Spine and Nerve Center of the Virginias in Charleston, W.Va. "Proclaim XR is a major advancement in spinal cord stimulation, and is an evidence-based therapy that is mobile app-based and features upgradeable software. This means patients won't need surgery to benefit from future advances in this technology."
Alternative stimulation systems may use higher frequencies or multiple waveforms that use more energy, requiring them to be recharged daily – taking time from a person's day. Abbott's Proclaim XR system allows physicians to identify the lowest effective dose of BurstDR stimulation customized to each patient, optimizing system longevity while maintaining effective pain relief, and eliminating the need for recharging (for up to 10 years at low-dose settings*). This patient-centric innovation is possible because of Abbott's proprietary waveform and advanced battery technology that is integrated into Proclaim XR.
"Proclaim XR is a new and differentiated advancement in how we approach chronic pain management, offering a safe and effective non-opioid technology for up to a decade of pain relief," said Jacqueline Weisbein, D.O., Napa Valley Orthopaedic Medical Group in Napa, Calif. "Having access to reliable pain relief – from a system that doesn't have the hassle of needing to be recharged –is a gamechanger in helping people live their best lives, free from pain."
Living With Chronic Pain On Her Terms
Sara Smith, 33, is a trained medical technician and loves the outdoors. As a young girl, she was diagnosed with scoliosis and, in 2006, she had spinal fusion surgery. Not deterred, she remained active and continued to hike, camp, and even ran a few marathons. Unfortunately, while riding a dirt bike in 2012, she had a serious accident that broke one of her spinal fusion bolts, which was the start of her debilitating chronic pain. Sara tried physical therapy, injection therapy and pain medications before being introduced to spinal cord stimulation therapy. In her clinical trial experience, she reported a reduction in pain by 75%, and was eager to receive her permanent implant as one of the first patients receiving Proclaim XR.
"I didn't realize how much pain I was really in until the Proclaim XR system trial," Smith said. "I was able to reclaim my life and can be the involved mom and wife I was before my accident. My Proclaim XR device's long battery life will allow me to be an active participant in my own life, without having to worry about finding someplace where I can charge my device. While I may never be completely pain free, my pain level is significantly reduced, allowing me to focus on the things that matter the most to me and my family."
Similar to Sara, approximately 50 million people in the US are affected by chronic pain, many of whom could benefit from spinal cord stimulation.
"Proclaim XR is the latest advancement in patient-centric pain therapy -- opening the doors for people who don't want to be burdened with the hassles of recharging. Recharge-free means patients can live their lives without the constant reminder of being in treatment," said Keith Boettiger, vice president of Abbott's neuromodulation business. "This is a meaningful advancement for the millions of people who need new options when it comes to managing the effects of their chronic pain. Abbott is committed to developing life-changing solutions and putting the patient at the center of everything we do."
Proclaim XR is currently under review for CE Mark.
Learn more about Proclaim XR and further clinical evidence to support Abbott's BurstDR stimulation. For important safety information, visit: abbott.com/isi.
About Abbott's Chronic Pain Portfolio
Chronic pain can negatively impact personal relationships, work productivity and a person's daily routine. Abbott is a global leader in the development of chronic pain therapy solutions, offering radiofrequency therapy and spinal cord stimulation therapy solutions, including BurstDR stimulation, and stimulation of the dorsal root ganglion in the portfolio of devices for the treatment of chronic pain.
The Proclaim XR system is the latest advancement from Abbott, featuring upgradeable software and consumer-friendly technology that is compatible with Apple devices such as the iPhone® or iPad® or iPod Touch®.
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 103,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com, on Facebook at www.facebook.com/Abbott and www.facebook.com/AbbottChronicPain on Twitter @AbbottNews and @AbbottGlobal.
* Up to 10 years of projected battery longevity at the lowest dose setting: 0.6mA, 500 Ohms, duty cycle 30s on/360s off. NOTE: In neurostimulation therapy, 'dose' refers to the delivery of a quantity of energy to tissue. Safety comparisons and specific dose-response curves for each dosage have not been clinically established. Refer to the IFU for additional information. Hassle-free means recharge-free. The BoldXR Dosing Protocol is only a guide and each patient should be programmed as needed to ensure the best outcome. It should not replace the IFU or recommendations and advisement of the treating health care practitioner.
SOURCE Abbott

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In











Share this article