Exero Medical Announces Enrollment of 100th Patient in Pivotal Study to Validate its Breakthrough Technology for Early Detection of Post Operative Complications
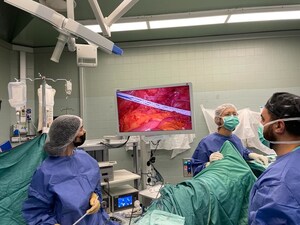
OR YEHUDA, Israel, Dec. 18, 2024 /PRNewswire/ -- The study, which includes eleven sites in the US and Israel, is enrolling patients undergoing colorectal resection surgery to validate the safety and accuracy of Exero's xBar system for early detection of major, life-threatening complications such as an anastomotic leaks. The outcome of the study will support Exero's FDA De Novo submission.
The xBar system consists of electrode sensors embedded in a standard surgical drain and placed near the anastomosis site. The electrodes are connected to a small electronic device outside the patient's body that records and relays data. The data is analyzed to indicate the risk of anastomotic leaks based on the status of tissue healing or damage.
The study commenced in March 2024, and the one hundredth patient was enrolled at Bryan Medical Center in Lincoln, Nebraska, by Dr. Brad Olberding, MD, General Surgeon.
Dr. Michael Jobst, Bryan Medical Center's Principal Investigator, explains, "The xBar sensor is placed through the standard procedure for surgical drains, and does not alter our routine surgical and post operative workflow. If proven successful, the data provided by xBar can support us with earlier detection of post operative complications. This could allow surgeons to intervene before the patient's condition becomes dire and help minimize prolonged hospital stays."
Dr. Erez Shor, Exero Medical's CEO, said, "This milestone marks significant progress toward the completion of xBar's pivotal trial, and brings us one step closer to making this technology available to circumvent life threatening complications, helping both patients and surgeons."
Further information can be found at Clinicaltrials.gov or https://exeromedical.com.
About Exero Medical
Exero Medical's mission is to empower health care providers with high-quality, actionable post-operative tissue healing data that can save lives, improve prognosis, and reduce cost of care. The company's flagship product, the xBar system, has been granted Breakthrough Device Designation by the FDA. Exero Medical was founded in 2018 by MEDX Xelerator, and is backed by the Israeli Innovation Authority, Longevity Venture Partners, Edge Medical Ventures, Clalit Health Services – the largest HMO in Israel, and Unorthodox Ventures.
The xBar system is not approved for sale in the United States and is limited to investigational use.
Media contact:
Eileen Ke
Vice President, Business Development
(+1) 267-342-0576
[email protected]
SOURCE Exero Medical

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article