- The largest commercial payer in Tennessee, representing 2.3 million covered lives, has established positive coverage—effective March 1, 2024—for both one- and two-level lumbar total disc replacement (TDR) procedures, which includes prodisc® L.
- With this positive coverage update in Tennessee, it is estimated that one-level lumbar TDR coverage is approaching 94%* of commercially-covered lives in the U.S., while two-level coverage continues to build with over 30% of U.S. covered lives.
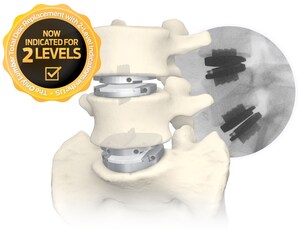
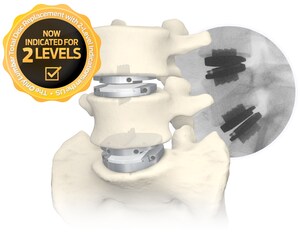
- Centinel Spine's prodisc L is the only lumbar TDR system in the U.S. approved for one- and two-level use in the lumbar spine.
WEST CHESTER, Pa., Feb. 21, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced significant expansion of coverage for lumbar TDR procedures in Tennessee.
The policy expansion announced by Tennessee's largest commercial payer—effective March 1, 2024—represents over 2.3 million covered lives across the state and includes a positive coverage recommendation for both one- and two-level lumbar TDR. Because prodisc L is the only lumbar TDR device with FDA approval for two-level use, the two-level coverage recommendation only applies to the use of the prodisc L lumbar device.
This major positive coverage update continues the trend toward significant coverage for one- and two-level lumbar TDR. In August 2023, the largest payers in Illinois, New Mexico, Oklahoma, and Texas—representing over 16 million covered lives—adopted Carelon's Clinical Appropriateness Guidelines-Musculoskeletal Spine Surgery Criteria, which deems one- and two-level lumbar TDR medically necessary. In September 2023, the largest commercial payer in Michigan added one- and two-level lumbar TDR coverage, providing 5.7 million covered lives with access to lumbar TDR. Then, in October 2023, the largest commercial payer in North Carolina adopted Carelon's Clinical Appropriateness Guidelines-Musculoskeletal Spine Surgery Criteria for lumbar TDR, as well—adding another 2.8 million covered lives.
With this favorable coverage expansion in Tennessee, it is estimated that coverage for one-level lumbar TDR has grown from 50% of covered lives in 2017 to now approaching 94%* today—and that over 30% of commercially-covered lives in the U.S. now have access to two-level lumbar TDR, as well.
Centinel Spine CEO Steve Murray shared, "The top commercial payer in Tennessee has now provided much needed one- and two-level lumbar total disc replacement access to a significant number of individuals. We have seen an acceleration of positive lumbar disc replacement coverage over the last 6 months. This is good for patients, and we believe this trend will continue as other payers recognize the clear and published clinical benefits of the total disc replacement procedure."
* Data of file, estimate based on combined positive and neutral policies.
About Centinel Spine, LLC
Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world.
The Company's prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 250,000 implantations. Centinel Spine's prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient's anatomy for both cervical and lumbar total disc replacement.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
SOURCE Centinel Spine, LLC

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article