- Record first quarter 2023 worldwide Motion Preservation revenue with over $14 million in sales, representing growth of 38% over first quarter 2022 and sequential growth of 10% over fourth quarter 2022, which was the previous best quarter.
- First quarter 2023 growth was driven by the limited U.S. launch of new cervical total disc replacement (TDR) technologies in September 2022, resulting in almost 900 procedures completed and over $6 million in sales since launch.
- Adoption of the new cervical TDR system accelerated in the first quarter 2023, as exhibited by 60% growth in average daily cases and almost 45% growth in additional new users versus prior quarter.
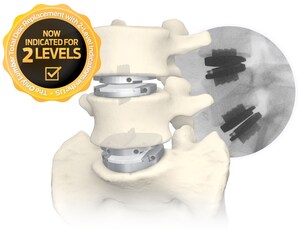
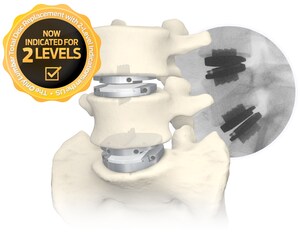
WEST CHESTER, Pa., May 18, 2023 /PRNewswire/ -- Centinel Spine®, LLC, ("the Company") a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-proven total disc replacement technology platform in the world (prodisc®), today announced record first quarter 2023 worldwide financial results in its Motion Preservation business. Record growth was primarily driven by continued strong adoption of the Company's new prodisc C Vivo and prodisc C SK cervical TDR system.
One of the recent new users of the new prodisc cervical system, neurosurgeon Tejpaul Pannu, MD of Michigan Head & Spine Institute, commented, "With the launch of the new prodisc C Vivo and prodisc C SK cervical TDR system, I now have the ability to match the particular disc I use to a variety of patient factors. The prodisc cervical system allows me to select a domed or flat implant coupled with either keel or spiked fixation. This optionality can be a benefit and is not available from any other total disc manufacturer."
Centinel Spine CEO Steve Murray shared, "We have seen a tremendous start to 2023, driven by the rapid uptake of prodisc C Vivo and prodisc C SK, which is the first TDR system to offer anatomic designs that match the disc to the needs of the surgeon and patient. We expect this momentum to continue throughout the year as we continue to expand availability of the new system and provide more access to surgeons and their patients."
First Quarter 2023 Highlights
First quarter 2023 revenue for the Company's worldwide Motion Preservation business exceeded $14 million (a record for the company), with growth of 38% over Q1 2022 and sequential growth of 10% over Q4 2022 (previous record quarter). Overall growth in the quarter was primarily driven by the September 2022 limited release of the new prodisc C Vivo and prodisc C SK cervical TDR system in the U.S. and continued global expansion in the prodisc L lumbar business. The U.S. Motion Preservation business grew 39% for the first quarter versus first quarter 2022, while the International Motion Preservation business grew 35%.
Since the Company initiated the limited U.S. launch of prodisc C Vivo and prodisc SK, rapid market acceptance of the new cervical TDR system has continued. Through the end of the first quarter 2023, almost 900 cases were completed with the new system, generating more than $6 million in sales with almost 200 new surgeon users. The adoption of the new cervical TDR system accelerated in the first quarter, as exhibited by 60% growth in average daily cases and almost 45% growth in additional new users versus the prior quarter. Over two-thirds of the surgeons using the new prodisc cervical system were repeat users, and an estimated 70% of users were competitive conversions. New surgeon users were added with the support of a global medical educational initiative that trained almost 160 surgeons in the quarter.
Strong commercial traction for the overall U.S. cervical and lumbar prodisc TDR business continued, demonstrated by total user growth of more than 20% over the first quarter 2022 and sequential growth exceeding 10% versus fourth quarter 2022. The number of U.S. facilities utilizing the Company's Motion Preservation products increased almost 30%, and the number of distributors selling the products expanded by upwards of 15% versus first quarter 2022.
Centinel Spine's prodisc portfolio represents the most comprehensive TDR systems in the world, offering both cervical and lumbar implants with anatomically-differentiated designs. Centinel Spine is the only company with FDA approval for both cervical and lumbar TDR systems. All of the prodisc cervical and lumbar devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 240,000 implantations worldwide.*
* Data on file.
About Centinel Spine, LLC
Centinel Spine®, LLC is a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access. The Company offers a continuum of trusted, brand-name solutions backed by over 30 years of clinical success—providing the most robust and clinically-proven technology platforms in the world.
Centinel Spine continues to advance its pioneering culture and corporate mission to become a catalyst of change in the spine industry and alter the way spine surgery is perceived. Centinel Spine's prodisc platform remains the only technology with multiple motion-preserving solutions for both cervical and lumbar anterior column reconstruction.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Centinel Spine
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
Media
Elizabeth Coleman
ICR Westwicke
Phone: 203-682-4783
Email: [email protected]
SOURCE Centinel Spine, LLC

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article