NEW YORK, Jan. 16, 2019 /PRNewswire/ -- Centinel Spine, LLC announced today that it has completed initial cases with its newest implant system in the FLX™ Platform of 3D-Printed All-Titanium Interbody devices - STALIF L FLX Lateral Lumbar Integrated Interbody™ System.
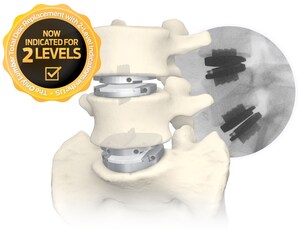
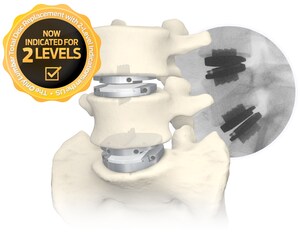
The STALIF L FLX Lateral Lumbar Integrated Interbody System represents the fourth system of 3D-Printed All-Titanium devices to be launched by Centinel Spine within the last 6 months, in addition to the STALIF C FLX™ Cervical Integrated Interbody, ACTILIF C FLX™ Cervical Interbody, and STALIF M FLX™ Lumbar Integrated Interbody devices. FLX devices feature a combination of solid and porous radiolucent sections designed to reduce mechanical stiffness and improve visibility, as compared to solid titanium implants. The devices also feature a proprietary FUSE-THRU™ trabecular scaffold, designed to allow for bony in-growth and on-growth throughout the implant.
"I have used numerous lateral systems – STALIF L is my number-one choice. The implants have a great fit and finish once placed, and the instrumentation is streamlined for ease-of-use," commented Dr. Christopher Cenac, Jr. of Gulf Coast Orthopedics, in Southeast Louisiana. "The new STALIF L FLX is the next step in strength and osteointegration. It continues Centinel Spine's march towards cutting edge innovation in the integrated interbody cage market."
The STALIF L FLX device offers a unique advantage over other lateral implant systems, as it features No Profile® integrated compressive lag screws, and is indicated for use at one or two contiguous levels with both autograft and/or allogenic bone graft.
"The availability of 3D-printed titanium lateral implants with zero profile fixation is exciting," says Dr. Mudit Sharma of Virginia Spine Specialists, "especially as more surgeons migrate towards titanium for decreasing instances of pseudarthrosis."
"The initial implantations of STALIF L FLX devices represent another major milestone in the evolution of our interbody technologies and advanced material platforms," says Centinel Spine Chairman & CEO, John Viscogliosi. "Our FLX products merge the proven benefits of the STALIF design with a truly novel, all-titanium lattice technology, and the availability of multiple material options in our lateral portfolio further advances our mission of becoming the global leader addressing spinal disease anteriorly with the widest breadth and depth of technology platforms."
Centinel Spine, the pioneer of the No Profile®, Integrated Interbody™ devices has more than 30 years of global clinical history and success behind these devices for the treatment of degenerative disc disease.
About Centinel Spine, LLC
Centinel Spine®, LLC is the largest privately-held spine company focused on anterior column reconstruction. The company offers a continuum of trusted, brand-name motion-preserving and fusion solutions backed by over 30 years of clinical success — providing the most robust and clinically-proven Total Disc Replacement and Integrated Interbody™ portfolios in the world.
The company began operations in 2008 through the merger-acquisition of two pioneering medical device companies—Raymedica, LLC and Surgicraft, LTD. UK-based Surgicraft commenced spine-focused operations in 1982 and launched the first Stand-Alone/No Profile® anterior lumbar interbody fusion device in the world in 1988. This first-of-its-kind device was the basis for future generations of the market-leading Integrated Interbody™ technology platform known today as STALIF®. Today, Centinel Spine still embraces the pioneering culture developed at both originating companies and continues its corporate mission to become the worldwide leading company addressing spinal disease anteriorly with the widest breadth & depth of technology platforms.
Centinel Spine recently acquired the prodisc® Total Disc Replacement Technology Platform—the most extensive cervical and lumbar motion-preserving reconstruction portfolio available today. With the addition of prodisc, Centinel Spine stands alone as the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction.
Centinel Spine's most recent technological advancement for its fusion devices includes the development and launch of the FLX™ Technology Platform. These 3D-printed, all-titanium devices feature a combination of solid and porous, radiolucent FUSE-THRU™ sections designed to reduce mechanical stiffness, improve visibility compared to solid titanium implants, and allow for bony on-growth and in-growth. The FLX technology is currently available in multiple implant portfolios including integrated and non-integrated cervical interbody systems, an integrated anterior lumbar interbody system, and an integrated lateral lumbar interbody system.
Centinel Spine derived its name from the "Sentinel Sign", the radiographic confirmation of a successful fusion anterior to the interbody device.
For more information on Centinel Spine products and technologies, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
SVP, Corporate Finance & Strategic Planning Centinel Spine, LLC
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
SOURCE Centinel Spine, LLC
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article