
Breakthrough Dental Device Detects Very Early Cavities Well Before X-Rays
New Ortek-ECD™ Developed and Tested at Stony Brook University School of Dental Medicine
ROSLYN HEIGHTS, N.Y., May 21, 2020 /PRNewswire/ -- Ortek Therapeutics, Inc. today announced the official US commercial launch of the Ortek-ECD™, a breakthrough electronic early cavity detection system that has been cleared by the FDA for professional use only. The ECD can detect cavity lesions on the surfaces of teeth that are too small to be detected by X-rays. Dental professionals can now treat cavities, one of the most chronic diseases in children and adults, before it can severely damage tooth structure.
The ECD was developed and tested at Stony Brook University School of Dental Medicine and demonstrated 100% sensitivity and 93% specificity in a peer-reviewed clinical study published in the Journal of Clinical Dentistry. The ECD is fast and easy to use, painless and does not use ionizing radiation. A stainless steel tip is gently placed in the grooves of molars and premolars, which are tooth surfaces most affected by decay. The ECD unit measures the conductivity of enamel and instantly displays a digital cavities score allowing dental professionals to recommend appropriate treatments, including minimally invasive procedures.
"Dental cavities afflict 60–90% of school-age children worldwide, and approximately 3 billion people suffer from untreated dental cavities," said Mitchell Goldberg, President of Ortek Therapeutics, Inc. "The ECD is a major technological advance and will significantly improve patient outcomes by conserving tooth structure and helping avoid the unwanted consequences of more advanced tooth decay."
The Technology
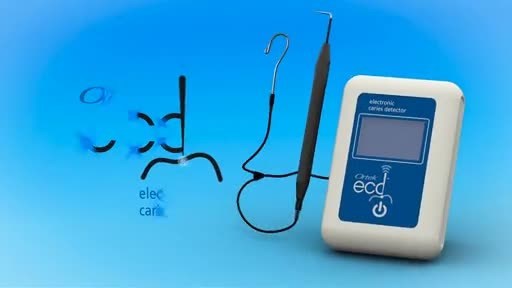
Powered by a 9 volt battery, the patented ECD is a small portable device that has a base unit with a digital display, a handpiece with a novel dimensionally configured conductive stainless steel tip that painlessly touches the bottom of a pit or fissure surface and a reference lip hook.
The revolutionary ECD measures the conductivity of enamel and was specifically designed to instantly detect very early tooth decay on the biting surfaces of molars and premolars. It's been estimated that 80%-90% of tooth decay occur in these vulnerable sites.
A tooth is considered cavitated if demineralization breaches the enamel and exposes the underlying dentin, which contains fluid filled tubules. When cavitation occurs, hydrostatic pressure will allow minuscule amounts of conductive dentinal fluid to enter the breached enamel site, allowing the ECD to complete an electrical circuit.
Loss of mineral from enamel as a result of cavity activity increases porous size and enamel porosity. As this demineralization increases, more dentinal fluid enters the breached site. The more fluid detected results in a higher current and an increasing digital caries score that is digitally displayed from 01-100. A zero score indicates a non-cavitated lesion or sound enamel site.
To learn more about the Ortek ECD, visit www.ecddetect.com
About Ortek
Ortek Therapeutics, Inc. is a global leader in developing and commercializing cutting edge oral care technologies. Through its long-standing strategic alliance with the Research Foundation for the State University of New York, Ortek has developed major scientific breakthroughs including the revolutionary arginine bicarbonate, calcium carbonate technology for cavity prevention and treatments for dentinal sensitivity. Additionally, this partnership has led to FDA clearance and commercialization for an advanced electronic cavity detection device - the Ortek ECD™.
SOURCE Ortek Therapeutics, Inc.


Share this article