- Nurtec ODT open-label extension study is the first of its kind evaluating a CGRP-targeted medication in patients who are using it as a preventive and an as-needed acute treatment for migraine
- Over the course of this open-label extension study, > 80% of patients experienced ≥50% reduction and approximately 50% experienced a 100% reduction
- Complete data from the Phase 3 zavegepant nasal spray study highlight its ultra-rapid pain relief in as little as 15 minutes that lasts through 48 hours after a single dose
- New studies showed positive efficacy, safety and pharmacokinetics data for Nurtec ODT as an acute treatment of migraine in adults from China and Korea
NEW HAVEN, Conn., June 13, 2022 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN) today announced new data from three late-breakers and three oral presentations supporting the safety and efficacy of Nurtec® ODT (rimegepant) and zavegepant nasal spray for the treatment of migraine. The oral presentations included a first of its kind study in migraine reporting on the safety and benefits of Nurtec ODT when used as a preventive treatment and as-needed for acute treatment of migraine. The data were presented at the 64th Annual Scientific Meeting of the American Headache Society (AHS) held from June 9-12 in Denver, CO.
Elyse Stock, MD, Chief Medical Officer of Biohaven commented, "Biohaven is proud of the growing body of clinical efficacy and safety data for Nurtec ODT and zavegepant, the building blocks of our CGRP franchise. We are committed to advancing research for migraine and bringing effective treatment options to the millions of patients living with it. We hope these data will give confidence to healthcare providers that Nurtec ODT can offer an effective, all-in-one treatment for patients to both prevent and treat migraine attacks. With the US FDA's recent acceptance of the NDA filing for zavegepant nasal spray, we are excited about its potential as a new therapy for the acute treatment of migraine."
These new data reinforce Nurtec ODT's efficacy and safety for patients using it to both prevent and treat migraine attacks. Biohaven also presented full Phase 3 efficacy and safety data for zavegepant nasal spray. The data show zavegepant nasal spray was effective for the acute treatment of migraine, achieving its coprimary endpoints and providing an ultra-rapid onset of pain relief at the earliest measured time point of 15 minutes and sustained benefits through 48 hours after a single intranasal dose, with favorable safety and tolerability.
Richard Lipton, MD, lead author and presenter of the 52-week, open-label Nurtec ODT extension study, and Professor and Vice Chair of Neurology at the Albert Einstein College of Medicine and Director of the Montefiore Headache Center commented, "I'm honored to share results from a first of its kind study – one that looked at Nurtec ODT's safety, tolerability, and outcomes when used as an acute and preventive treatment of migraine over 52 weeks after the 12-week double-blind prevention study. Given these positive outcomes coupled with a consistently safe and well-tolerated profile, the data continue to suggest that Nurtec ODT represents an effective, flexible treatment option for patients."
The oral presentations spotlighting the new data were presented on Saturday, June 11. Details from all late-breaker and oral presentations are shared below.
Late-Breakers
- Patterns of Medication Utilization and Migraine Frequency in Adults Using Rimegepant for Both Preventive and Acute Treatment for Migraine: Results from a 52-Week, Open-Label Extension Study
- A 52-week, open-label extension study of rimegepant 75 mg dosed every other day for preventive treatment plus as-needed for acute treatment of migraine demonstrated that the monthly frequency of moderate or severe migraine days and use of other acute migraine medications were uncommon and consistent between scheduled versus nonscheduled rimegepant dosing days, suggesting that the benefits of rimegepant are sustained. On nonscheduled dosing days, rimegepant was used as an acute treatment approximately 1 day per month, and additional acute medications were rarely utilized.
- Efficacy, Safety, and Tolerability of Rimegepant 75 mg Orally Disintegrating Tablet for the Acute Treatment of Migraine: Results from a Phase 3, Double-Blind, Randomized, Placebo-Controlled Trial in Adults From China and Korea (Study BHV3000-310)
- In a large Phase 3 trial evaluating the safety and efficacy of rimegepant for the acute treatment of migraine in 1,431 patients in the People's Republic of China and the Republic of Korea, rimegepant was determined to be superior to placebo for both coprimary endpoints: pain freedom at 2 hours post-dose (19.8% vs 10.7%, P<.0001) and freedom from most bothersome symptom (MBS) (50.5% vs 35.8%, P<.0001). A single dose of rimegepant 75 mg, without rescue medication, was superior to placebo demonstrating sustained pain relief from 2 hours post-dose through 48 hours post-dose.
- A Phase 1, Randomized, Placebo-controlled, Single- and Multiple- dose, Double-blind Study to Evaluate the Pharmacokinetics and Safety of Rimegepant Orally Disintegrating Tablets 75 mg in Healthy Chinese Adults
- This Phase 1 study evaluated safety and pharmacokinetics of rimegepant in 16 patients of Chinese ethnicity. Overall, administration of single and multiple rimegepant 75 mg doses was safe and well tolerated in healthy Chinese adults. The PPK model supports the rimegepant 75 mg dose in the Chinese population for the acute treatment of migraine, repeated up to once daily, with the conclusion that there are no significant ethnicity differences of rimegepant PK between Chinese and non-Chinese participants.
Oral and Poster Presentations
- Safety and Tolerability of Rimegepant Every Other Day for Preventive Treatment of Migraine Plus As-Needed for Acute Treatment of Migraine: Results from a 52-Week, Open-Label Extension Study
- In a long-term, open-label extension phase of the Phase 2/3 study of rimegepant 75 mg as a preventive treatment of migraine in adults, 603 patients were administered rimegepant every other day plus as-needed on nonscheduled dosing days for up to one year. The data showed that rimegepant 75 mg is safe and well tolerated with no liver safety concerns. The most common on-treatment adverse events (AEs) were upper respiratory tract infection (7.1%), nasopharyngitis (6.3%), and back pain (4.3%). The rate of discontinuation due to AEs was 2.8%. Though serious AEs occurred in 2.2% of subjects, none were considered to be related to rimegepant.
- A separate poster presentation of this study presented analyses of reductions in migraine frequency over time. Over the course of the study, 80.9% of patients experienced ≥50% reduction in moderate or severe monthly migraine days, 65.8% experienced a ≥75% reduction, and 49.3% experienced a 100% reduction.
- Efficacy and Safety of Zavegepant Nasal Spray for the Acute Treatment of Migraine: Results of a Phase 3 Double-Blind, Randomized, Placebo Controlled Trial
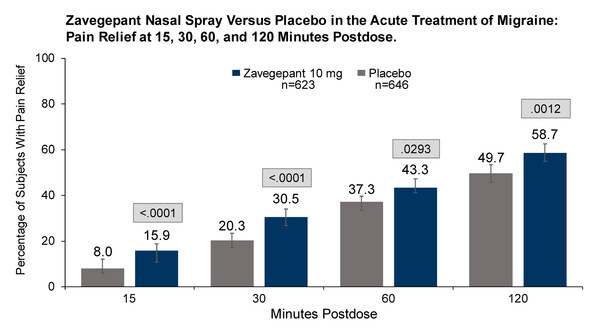
- Complete results from this Phase 3 clinical trial evaluating the efficacy and safety of zavegepant nasal spray in 1,405 randomized patients reported that zavegepant is effective for the acute treatment of migraine with favorable safety and tolerability. Zavegepant was superior to placebo for the coprimary endpoints: freedom from pain 2 hours post-dose (23.6% vs 14.9%, P<.0001) and freedom from most bothersome symptom 2 hours post-dose (39.6% vs 31.1%, P=.0012). Secondary endpoints included pain relief at 15 minutes (15.9% vs 8.0%, P<.0001) and 2 hours (58.7% vs 49.7%, P=.0012); return to normal function at 30 minutes (10.5% vs 6.1%, P=.0059) and 2 hours (35.8% vs 25.6%, P=.0001); and sustained pain relief 2 to 48 hours (36.1% vs 29.6%, P=.013) post-dose.
- Increased Placebo Response Over Time in Oral Migraine Preventive Trials: A Systematic Literature Review and Meta-analysis
- This study evaluated trends in placebo response over time in preventive migraine treatment clinical trials from January 1990 to August 2021. The researchers observed a significant increase in oral placebo response across time, indicating that routes of administration (oral and injectable) may exhibit different levels of contextual effect. Additionally, increasing temporal trends in placebo response may bias future meta-analyses intended to indirectly compare the effectiveness of preventive migraine medications where the time span of included trials is large. This can also impact study design and approval of future migraine treatments due to decreasing effect sizes.
Dr. Lipton is a paid consultant and stockholder of Biohaven.
About NURTEC ODT
NURTEC ODT (rimegepant) is the first and only calcitonin gene-related peptide (CGRP) receptor antagonist available in a quick-dissolve ODT formulation that is approved by the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine with or without aura and the preventive treatment of episodic migraine in adults. The activity of the neuropeptide CGRP is thought to play a causal role in migraine pathophysiology. NURTEC ODT is a CGRP receptor antagonist that works by reversibly blocking CGRP receptors, thereby inhibiting the biologic activity of the CGRP neuropeptide. The recommended dose of NURTEC ODT is 75 mg, taken as needed, up to once daily to treat or every other day to help prevent migraine attacks. For more information about NURTEC ODT, visit www.nurtec.com. The most common adverse reaction was nausea and abdominal pain/indigestion. Avoid concomitant administration of NURTEC ODT with strong inhibitors of CYP3A4, strong or moderate inducers of CYP3A. Avoid another dose of NURTEC ODT within 48 hours when it is administered with moderate inhibitors of CYP3A4 or potent inhibitors of P-gp.
Indication
NURTEC ODT orally disintegrating tablets is a prescription medicine that is used to treat migraine in adults. It is for the acute treatment of migraine attacks with or without aura and the preventive treatment of episodic migraine. It is not known if NURTEC ODT is safe and effective in children.
Important Safety Information
Do not take NURTEC ODT if you are allergic to NURTEC ODT (rimegepant) or any of its ingredients.
Before you take NURTEC ODT, tell your healthcare provider (HCP) about all your medical conditions, including if you:
- have liver problems,
- have kidney problems,
- are pregnant or plan to become pregnant,
- are breastfeeding or plan to breastfeed.
Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
NURTEC ODT may cause serious side effects including allergic reactions, trouble breathing and rash. This can happen days after you take NURTEC ODT. Call your HCP or get emergency help right away if you have swelling of the face, mouth, tongue, or throat or trouble breathing. This occurred in less than 1% of patients treated with NURTEC ODT.
The most common side effects of NURTEC ODT were nausea (2.7%) and stomach pain/indigestion (2.4%). These are not the only possible side effects of NURTEC ODT. Tell your HCP if you have any side effects.
You are encouraged to report side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1–800–FDA–1088 or report side effects to Biohaven at 1–833–4NURTEC.
See full Prescribing Information and Patient Information.
About Zavegepant
Zavegepant is a third generation, high affinity, selective and structurally unique, small molecule CGRP receptor antagonist from Biohaven's NOJECTION™ Migraine Platform and the only CGRP receptor antagonist in clinical development with both intranasal and oral formulations. The efficacy and safety profile of intranasal zavegepant for the acute treatment of migraine, as compared to placebo, was shown in a randomized controlled Phase 2/3 dose-ranging trial with a total of over 1000 patients who received zavegepant. In this study, zavegepant showed statistical superiority to placebo on the coprimary endpoints of 2-hour freedom from pain and freedom from a patients' most bothersome symptom (either nausea, photophobia or phonophobia). This was the second zavegepant pivotal clinical trial to meet these coprimary endpoints. The U.S. Food and Drug Administration has accepted a new drug application for zavegepant with a Prescription Drug User Fee Act date in the first quarter of 2023.
About Biohaven
Biohaven is a global commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's Neuroinnovation™ portfolio includes FDA-approved NURTEC® ODT (rimegepant) for the acute and preventive treatment of migraine (EMA-approved as VYDURA® for the acute treatment of migraine with or without aura, and prophylaxis of episodic migraine in adults who have at least four migraine attacks per month) and a broad pipeline of late-stage product candidates across five distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine and other CGRP-mediated diseases; glutamate modulation for obsessive-compulsive disorder and spinocerebellar ataxia; myeloperoxidase (MPO) inhibition for amyotrophic lateral sclerosis; Kv7 ion channel activators for focal epilepsy and neuronal hyperexcitability, and myostatin inhibition for neuromuscular diseases. More information about Biohaven is available at www.biohavenpharma.com.
NURTEC, NURTEC ODT and VYDURA are registered trademarks of Biohaven Pharmaceutical Ireland DAC. Neuroinnovation and NOJECTION are trademarks of Biohaven Pharmaceutical Holding Company Ltd.
Forward-looking Statement
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "believe", "continue", "may", "will", "anticipate", "expect" and similar expressions, are intended to identify forward-looking statements. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of Biohaven's management about NURTEC ODT as an acute treatment for patients with migraine and preventive treatment for migraine. Factors that could affect these forward-looking statements include those related to: Biohaven's ability to effectively commercialize NURTEC ODT, delays or problems in the supply or manufacture of NURTEC ODT, complying with applicable U.S. regulatory requirements, the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; the potential commercialization of Biohaven's product candidates; the potential for Biohaven's product candidates to be first in class or best in class therapies; and the effectiveness and safety of Biohaven's product candidates. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of the Company's Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 25, 2022, and in Biohaven's subsequent filings with the Securities and Exchange Commission. The forward-looking statements are made as of the date of this new release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Biohaven Contact
Jennifer Porcelli
201-248-0741
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
Media Contact
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502
SOURCE Biohaven Pharmaceutical Holding Company Ltd.

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In

Share this article