SEOUL, South Korea, May 13, 2021 /PRNewswire/ -- Hyundai Bioscience (KOSDAQ 048410) announced that Dr. Jin-Ho Choi, a Chief Scientist of its major shareholding bio tech company CNPharm and Chair Professor at Dankook University, presented the study titled 'COVID-19 Game Changer Drug – a novel organic-inorganic hybrid oral formulation, Niclosamide-dehydrotalcite' at the 20th Science Council of Asia (SCA) Conference held in Guangzhou, China, on May 13th. The presentation included the efficacy test results and related data from CP-COV03, an oral treatment for COVID-19 developed by CNPharm, on SARS-CoV2 infected animals at the Korea Zoonosis Research Institute (KoZRI), Jeonbuk National University in Korea.
According to the in vivo study that compared viral loads in the blood of the control group and treated groups, the control group exhibited its viral load in the blood to be recorded the highest on the 3rd day of infection and started to decrease from the 4th day, while all five treated groups recorded the lowest level on the same day. In particular, in the group administered with the lowest dose of 25mg/kg among the treated groups, a clear antiviral effect was confirmed such that the number of virus copies was '0' or close to '0'. (See graph 1)
The decreased viral load in the treated group results from Niclosamide's antiviral mechanism in both suppressing viral replication and deactivating the virus (virucidal). This study may be the world's first in vivo evidence for anthelminthic Niclosamide to be repurposed as an antiviral drug in oral formulation.
CNPharm's CP-COV03 is now expected to compete with other COVID-19 antiviral oral drug candidates, such as Pfizer's PF-07321332 and Merck's MK-4482. CNPharm expects that Niclosamide-based CP-COV03 would exhibit better antiviral efficacy, considering Niclosamide's dual antiviral mechanism of inhibiting replication and destroying the virus.
Antiviral drugs commonly have a mechanism to inhibit the replication of the virus, like Tamiflu which was considered the 'game changer' for H1N1 influenza, and they are different from virucidal drugs that deactivate or destroy viruses usually with their toxicity. Since Tamiflu was developed for H1N1, it has been recognized that cutting edge biotechnology is required to develop such antiviral drugs successfully. That's why only a few big pharma companies, Pfizer or Merck for example, have been able to develop antiviral oral drugs and reach clinical stages for COVID-19 treatment.
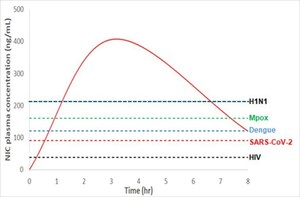
Dr. K.I. Kim, CNPharm's chief technical officer, explains that "unlike other types of drugs, antiviral drug project requires evaluating the maximum drug concentration level in the blood (Cmax) and the duration of maintaining effective concentration level for 50% viral inhibition (IC50)". Dr. Kim says "the broader the gap between the Cmax and IC50, the higher chance of a successful antiviral drug".
Merck's clinical study shows that MK-4482 maintained IC50 for more than 8 hours with a single dose and its Cmax level was 22 times higher than its IC50 level. According to CNPharm's in vivo animal model study, CP-COV03's IC50 level lasted more than 24 hours with a single dose, and its Cmax level was approximately 300 times its IC50 level, which showed great promise for an effective antiviral drug.
Hyundai Bioscience and CNPharm project that CP-COV03 could become the first drug repurposing of Niclosamide as an antiviral oral drug and a 'game changer' for the COVID-19 pandemic, and both firms are in preparation for the launch of clinical trials.
Niclosamide, since it was first discovered as anthelmintic in 1958, has been widely acknowledged as universal antiviral agent with efficacy against corona viruses such as SARS-CoV (severe acute respiratory syndrome) coronavirus and MERS (middle east respiratory syndrome) coronavirus, and RNA viruses such as Ebola and Zika viruses. Niclosamide was also selected as one of the most promising candidates for effective and safe treatment of COVID-19 in recent drug screening studies.
Niclosamide had been known for its low bioavailability and short half-life in plasma concentration, which made it too hard to be repurposed. CNPharm developed CP-COV03 to repurpose Niclosamide with its drug delivery system technology, and successfully improved bioavailability and the half-life of Niclosamide. CP-COV03 is anticipated to be a game changer for COVID-19 as well as other future viral diseases.
-END-
About Hyundai Bioscience:
HYUNDAI BIOSCIENCE is a biotechnology company that develops and commercializes bio-fusion technology with the purpose of delivering active ingredients safely and efficiently to targeted areas of the body. Hyundai Bioscience's Organic-Inorganic Hybrid Technology is a novel drug delivery system technology that allows the selective and effective delivery of active substances on the target area in combination with bio-friendly delivery vehicles. Applying its technology in the fields of tumor-targeted drugs and even skin & scalp care, Hyundai Bioscience will continue to expand its business scope by introducing innovative biotechnology and new effective products.
SOURCE Hyundai Bioscience

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article