American Association for Cancer Research Releases Cancer Progress Report 2017
Immunotherapy and precision medicine continue to be at the forefront of progress
Editor's Note: Please contact us for b-roll or to set up expert interviews. Contact Julia Gunther, [email protected] or 215-446-6896.
PHILADELPHIA, Sept. 13, 2017 /PRNewswire/ -- Federally funded research that provides a deep understanding of cancer is spurring advances against many types of the disease. With a strong bipartisan commitment from Congress to keep investment in biomedical research a national priority, we can accelerate our pace of progress and save more lives from cancer, according to the seventh annual American Association for Cancer Research (AACR) Cancer Progress Report, released today.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8155051-aacr-cancer-progress-report-2017/
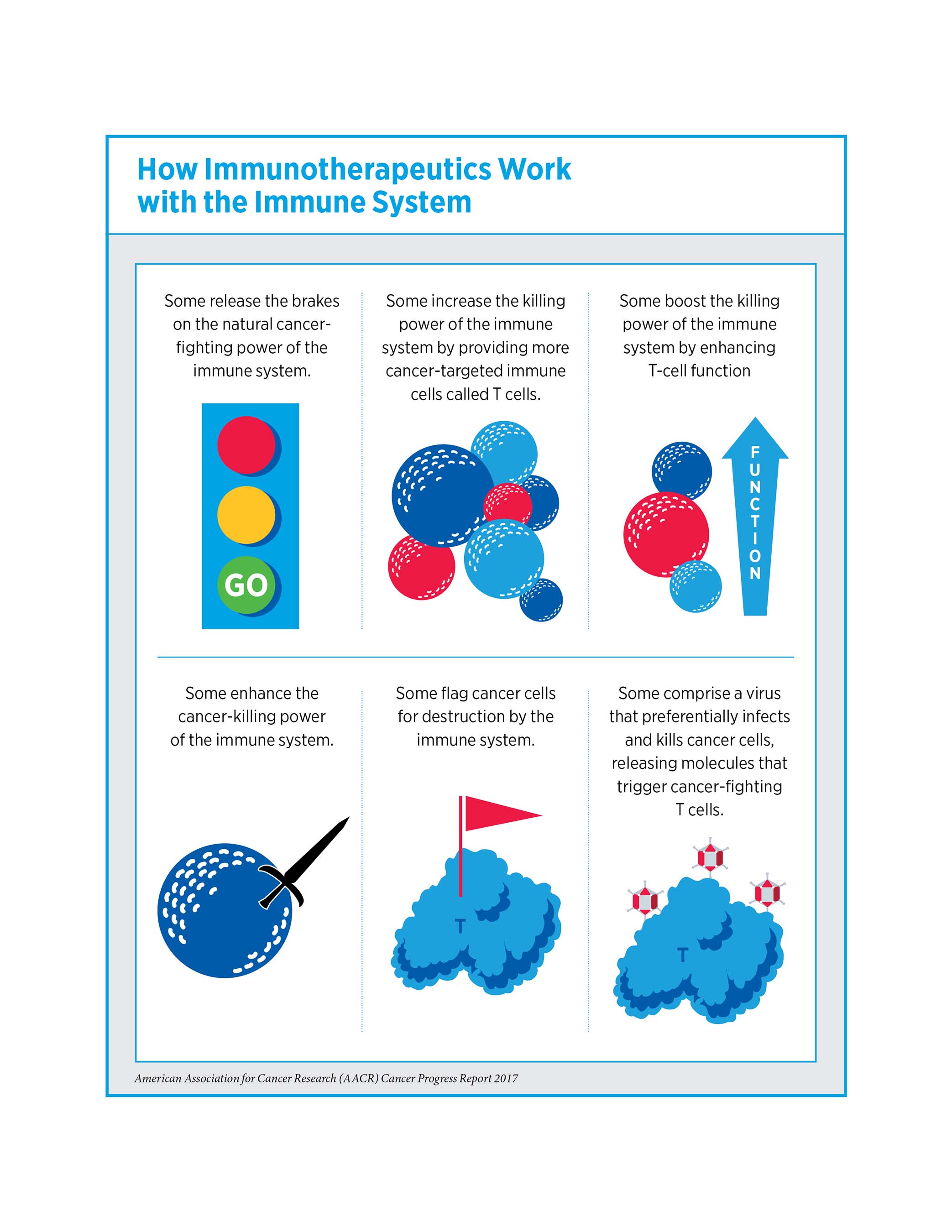
Basic research in the fields of immunology and cancer genetics has recently been harnessed to develop two new forms of cancer treatment: immunotherapy and precision medicine. As detailed in the report, the utility of these treatments is expanding rapidly. In May 2017, the U.S. Food and Drug Administration (FDA) heralded a new dawn for precision medicine when it approved the immunotherapeutic pembrolizumab (Keytruda) for treating patients with any solid tumor harboring specific genetic characteristics. This is the first anticancer therapeutic approved based on cancer biomarkers rather than the location in the body where the cancer originated.
"As research has taught us more about the biology of cancer, we have made incredible advances in cancer treatment and prevention that are saving lives today," said Michael A. Caligiuri, MD, President of the AACR, director of the Ohio State University Comprehensive Cancer Center, and chief executive officer of the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute in Columbus, Ohio. "The opportunity to make more transformational breakthroughs will require a strong federal commitment to providing consistent, annual, above-inflation increases to the budgets for the NIH [National Institutes of Health], NCI [National Cancer Institute], and FDA."
The annual AACR Cancer Progress Report is a cornerstone of the AACR's efforts to increase public understanding of cancer and the importance of cancer research to improved public health, while also advocating for increased funding for government agencies that fuel progress against cancer, in particular the NIH, NCI, and FDA.
Research: Driving Progress Against Cancer
The AACR Cancer Progress Report 2017 details how individuals working across the spectrum of cancer research from basic to translational to clinical and population research are fueling the development of new ways to prevent, detect, diagnose, and treat cancer. They are providing new hope to cancer patients, survivors, and their family members and friends, including the eight individuals who have shared their personal experiences with cancer in the report.
Progress highlighted in the AACR Cancer Progress Report 2017 includes the following:
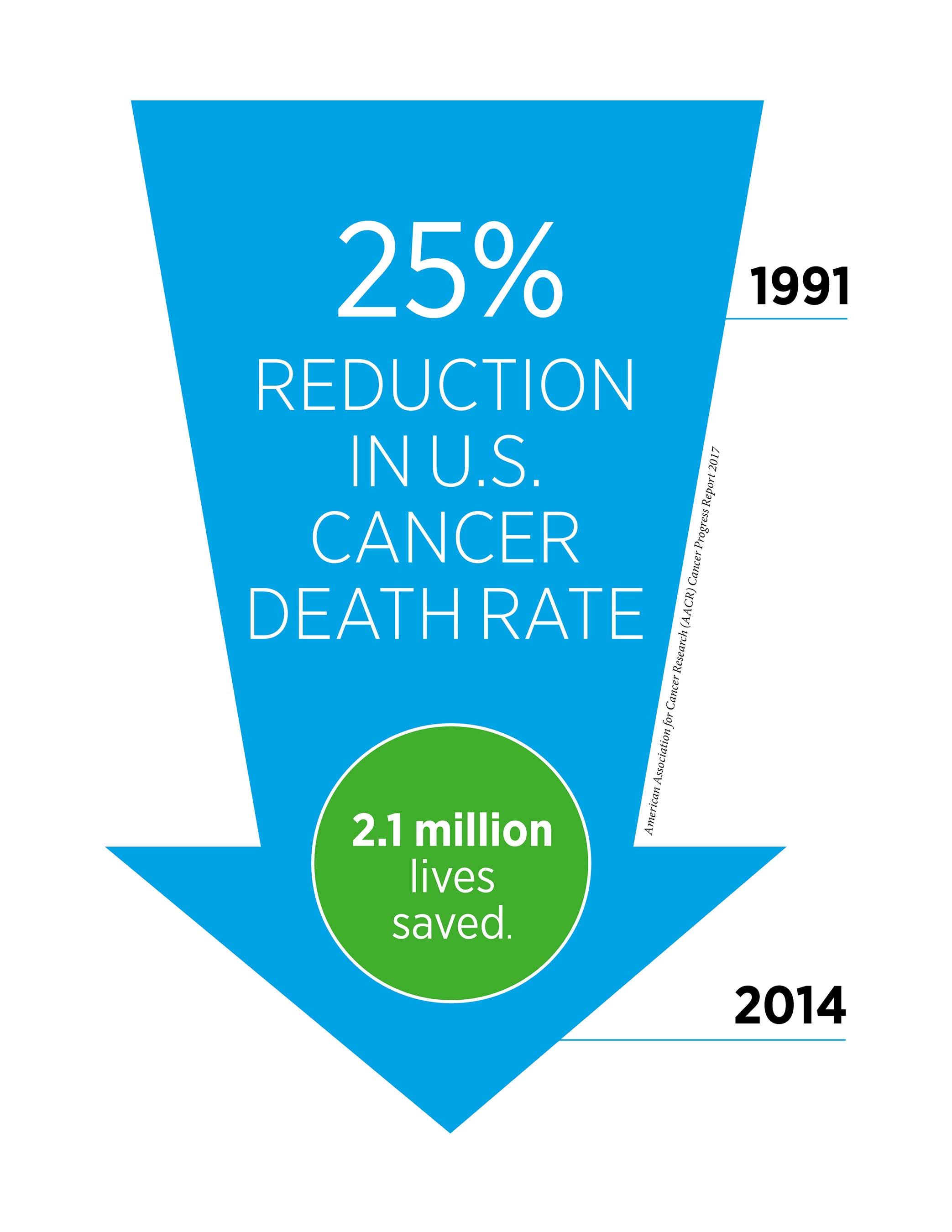
- According to the latest data, the U.S. cancer death rate declined by 35 percent from 1991 to 2014 for children and by 25 percent for adults, a reduction that translates into 2.1 million cancer deaths avoided.
- Between Aug. 1, 2016, and July 31, 2017, the FDA approved nine new anticancer therapeutics and eight previously approved anticancer therapeutics for treating new types of cancer.
- Two of the new anticancer therapeutics are in a class of immunotherapeutics called checkpoint inhibitors, revolutionary treatments that are increasing survival and improving quality of life for patients with an increasing number of types of cancer.
- Research discoveries continue to advance precision medicine: Seven of the new anticancer therapeutics are molecularly targeted agents.
- During this period, the FDA also approved one new optical imaging agent to help visualize gliomas and ensure more complete surgical removal of these brain tumors.
- Thanks to public education and policy initiatives, total U.S. adult cigarette consumption, which is the leading cause of lung cancer, decreased by 38.7 percent from 2000 to 2015.
Cancer: An Ongoing Challenge and Costly Disease. Research: A Vital Investment
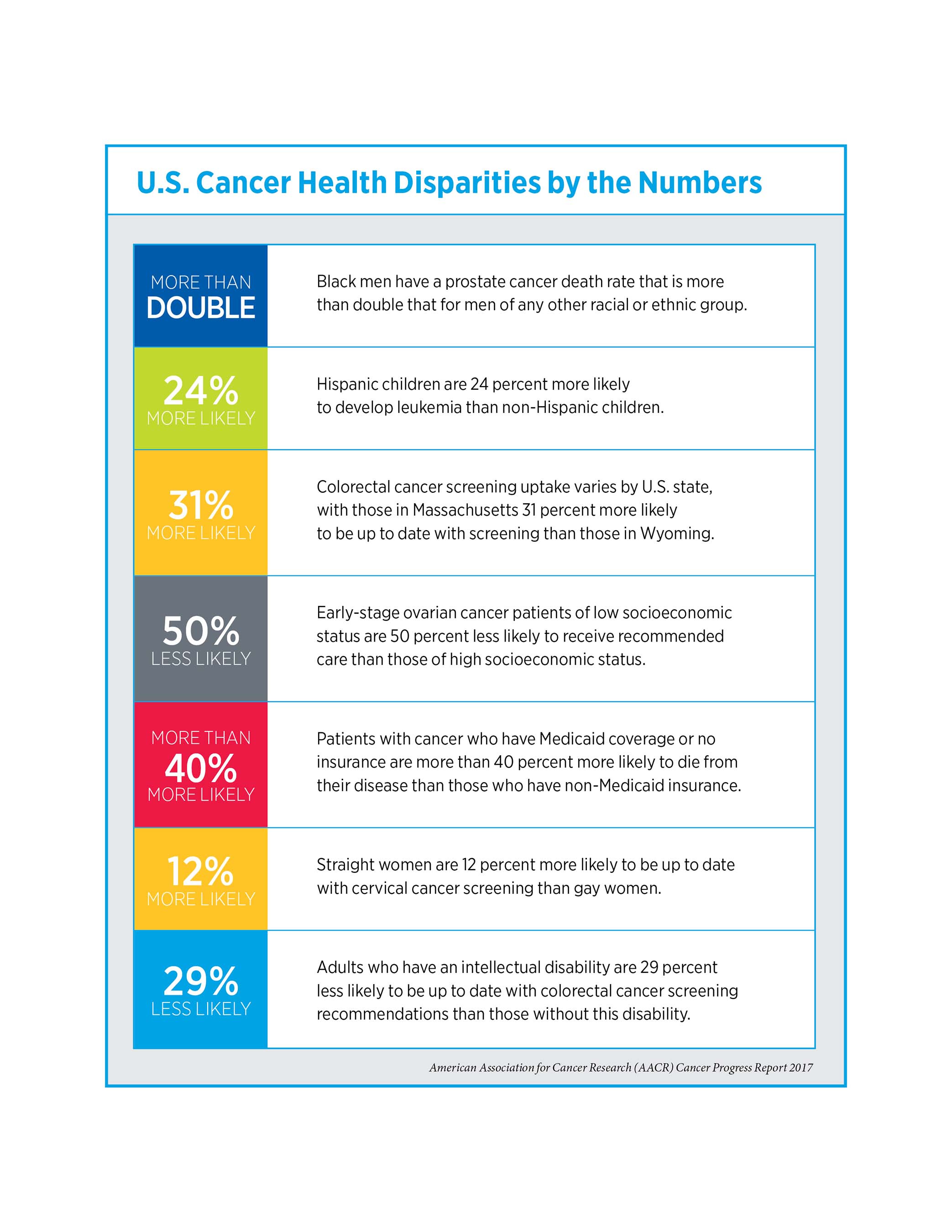
The report emphasizes that even though significant advances have been made, cancer continues to exert an immense personal and economic toll, and that the burden of cancer is shouldered disproportionately by certain segments of the population, including racial and ethnic minorities, patients of lower socioeconomic status, residents in certain geographic locations, and the elderly.
According to the report:
- More than 600,920 people in the United States are projected to die from cancer in 2017.
- The number of new cases of cancer in the United States is predicted to rise from 1.7 million in 2017 to 2.3 million in 2030.
- HPV vaccination could prevent nearly all cases of cervical cancer, as well as many cases of oral and anal cancer, but only 63 percent of girls and less than 50 percent of boys had received at least one dose of HPV vaccine in 2015.
- Advances against cancer have not benefited everyone equally and cancer health disparities are some of the most pressing challenges posed by the disease.
- It is estimated that the direct medical costs of cancer care in the United States in 2014 were nearly $87.6 billion; this number, which does not include the indirect costs of lost productivity due to cancer-related morbidity and mortality, stands in stark contrast to the budget that the NIH received that same year, which was $30.1 billion.
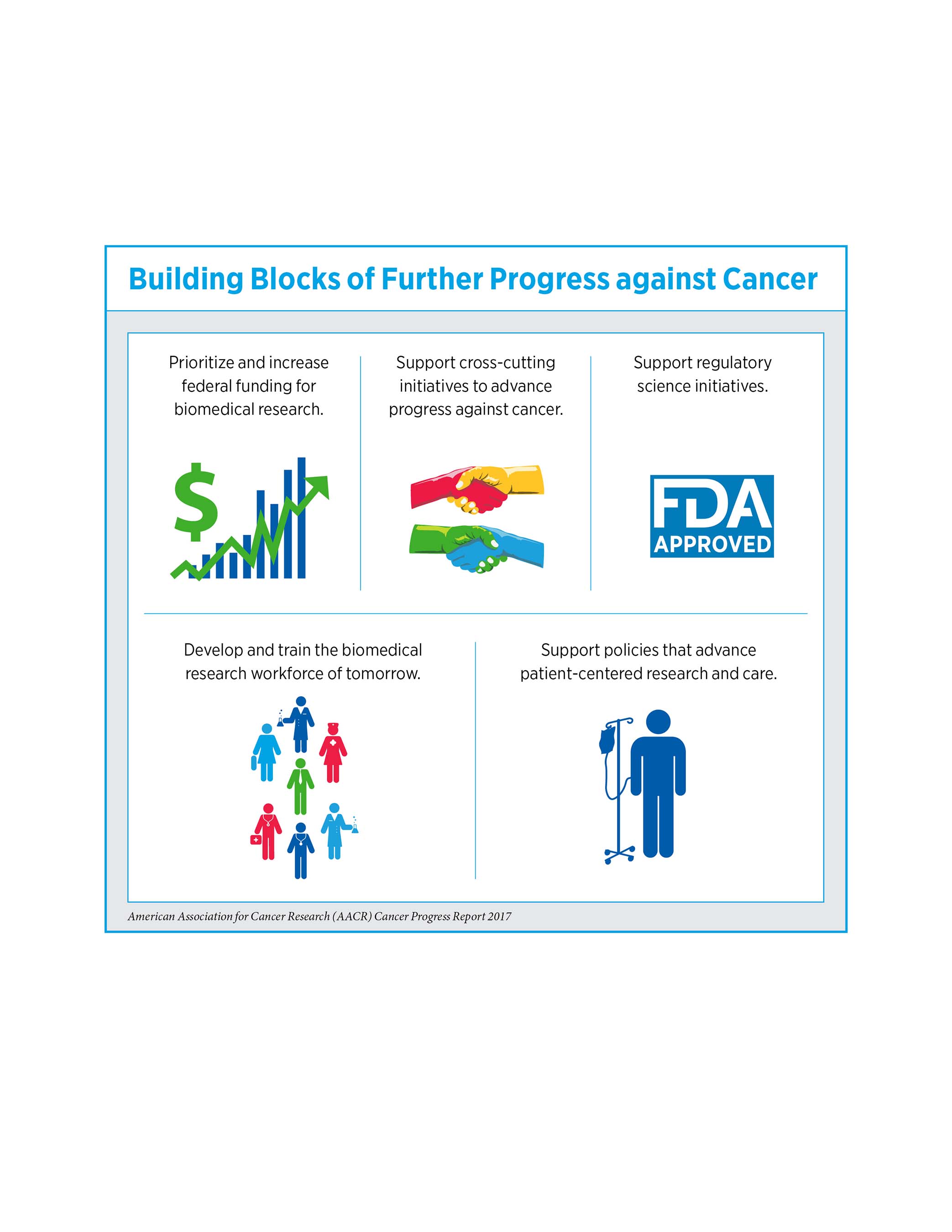
The report states that the increasing economic and personal burden of cancer underscores the need for more research to develop new approaches to cancer prevention and treatment. It also calls for Congress to:
- Continue to support robust, sustained, and predictable growth of the NIH budget by providing an increase of $2 billion for NIH in fiscal year (FY) 2018, for a total funding level of $36.2 billion.
- Ensure that funding designated through the 21st Century Cures Act for initiatives and programs, such as the Beau Biden Cancer Moonshot and the FDA Oncology Center of Excellence, is fully appropriated in FY 2018.
- Increase the FDA budget in FY 2018 to $2.8 billion, an $80 million increase above its FY 2017 level, to ensure support for regulatory science and to accelerate the pace of development of medical products that are safe and effective.
- Negotiate a bipartisan budget deal to raise the discretionary budget caps for FY 2018 and beyond, which would allow our nation's policy makers to continue to invest in priority areas, such as biomedical research funded by the NIH.
"This is an incredibly exciting time for the cancer community," said Margaret Foti, PhD, MD (hc), chief executive officer of the AACR. "Research has fueled advances across the continuum of cancer care that are saving lives around the world and we have the scientific knowledge and capability to deliver more lifesaving progress in the future.
"The AACR is heartened by the strong, bipartisan commitment from Congress to invest in cancer research and biomedical science," continued Foti. "We are committed to collaborating with all stakeholders to ensure that the current momentum is sustained and to expedite the next breakthroughs against cancer."
Follow us: Cancer Research Catalyst http://blog.aacr.org; Twitter @AACR; and Facebook http://www.facebook.com/aacr.org
For AACR information, visit Fast Facts.
About the American Association for Cancer Research
Founded in 1907, the American Association for Cancer Research (AACR) is the world's first and largest professional organization dedicated to advancing cancer research and its mission to prevent and cure cancer. AACR membership includes more than 37,000 laboratory, translational, and clinical researchers; population scientists; other health care professionals; and patient advocates residing in 108 countries. The AACR marshals the full spectrum of expertise of the cancer community to accelerate progress in the prevention, biology, diagnosis, and treatment of cancer by annually convening more than 30 conferences and educational workshops, the largest of which is the AACR Annual Meeting with more than 21,900 attendees. In addition, the AACR publishes eight prestigious, peer-reviewed scientific journals and a magazine for cancer survivors, patients, and their caregivers. The AACR funds meritorious research directly as well as in cooperation with numerous cancer organizations. As the Scientific Partner of Stand Up To Cancer, the AACR provides expert peer review, grants administration, and scientific oversight of team science and individual investigator grants in cancer research that have the potential for near-term patient benefit. The AACR actively communicates with legislators and other policymakers about the value of cancer research and related biomedical science in saving lives from cancer. For more information about the AACR, visit www.AACR.org.
Media Contacts:
Julia Gunther
[email protected]
215-446-6896
Rachel Salis-Silverman
[email protected]
215-446-7159
SOURCE American Association for Cancer Research
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In


Share this article