
The newest osmometer designed to meet complex bioprocessing needs
NORWOOD, Mass., Aug. 1, 2019 /PRNewswire/ -- Advanced Instruments—an innovative developer of scientific and analytical instruments and related services for the biotech industry—announced the latest release to its biotech instrument portfolio, the OsmoTECH PRO Multi-Sample Micro-Osmometer.
Testing osmolality is an essential parameter for process control and QC – just one of the many complex operational steps in biopharma manufacturing dedicated to producing a biologic drug of predetermined yield, purity and quality. The OsmoTECH PRO meets this crucial checkpoint on several fronts. Extensive data security features were developed in order to support compliance, most notably 21 CFR Part 11 and EU Annex 11. OsmoTECH PRO also is designed to meet osmolality testing pharmacopeia guidelines, and is an accurate, precise and easy-to-use device that fits well into existing production workflows.
OsmoTECH PRO was designed with extensive biotech customer feedback, according to Byron Selman, President and CEO of Advanced Instruments. "It is important for the OsmoTECH PRO to fit into today's bioprocessing workflows, and have the necessary technology to allow it to adapt in ever-evolving processes. Thanks to having some of the most robust data management and integrity features available on the market, OsmoTECH PRO is designed to meet those customer needs."
The OsmoTECH PRO has the most data-management features built into any osmometer currently on the market. Users can choose from several configuration settings to evolve during R&D, preclinical research, clinical testing and GMP compliance stages.
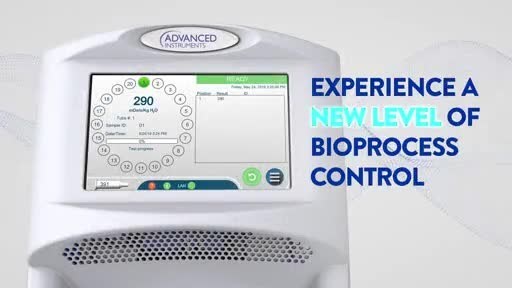
In addition to advanced data management and security features, OsmoTECH PRO promises user friendliness, thanks to an intuitive touch screen and factory-calibrated readiness. A 20-position sample turntable and small sample size requirement (only 30µL) also allow labs to get up and running quickly.
The OsmoTECH PRO utilizes freezing point depression – the gold standard method for testing osmolality. It is a truly comprehensive measurement of varied samples, as it's independent of size, ionization status, shape and other physical characteristics of the liquid solutions it measures.
About Advanced Instruments
Advanced Instruments is a global provider of scientific and analytical instruments for the biotechnology, clinical, and food-and-beverage industries. Since 1955, the company's innovations have helped organizations improve quality of results, achieve reliable outcomes, and increase workplace productivity. Advanced Instruments has a diverse portfolio of products, including: freezing-point osmometers, cerebrospinal fluid cell counters, anaerobic jar systems, cryoscopes, pasteurization test systems, and testing standards and controls. For more information, visit aicompanies.com.
OsmoTECH PRO is not for patient diagnostic use. Advanced Instruments certifies that the technical features needed for 21 CFR Part 11 and EU Annex 11 compliance are built into OsmoTECH PRO. It is your responsibility to implement the necessary controls in your laboratory to comply with 21 CFR Part 11 and EU Annex 11 requirements.
SOURCE Advanced Instruments


Share this article