BRAIN.Q Achieves CE Mark for Its Breakthrough Therapy for Reducing Disability Following Stroke and Welcomes Stacey Pugh as Chairman of the Board
ENGLEWOOD CLIFFS, N.J., Jan. 13, 2025 /PRNewswire/ -- BRAIN.Q, is excited to announce the CE Mark for its BQ 2.0 system, designed to reduce disability following ischemic stroke, the number one cause of disability worldwide.
Reducing stroke disability is often achieved through endovascular clot removal in the acute phase, during the first 12 hours after a stroke. This regulatory approval marks the first therapy to show a reduction in global disability outside of that acute window. Further, the company is currently enrolling in the EMAGINE II pivotal study in the U.S. with the aim of achieving FDA clearance.
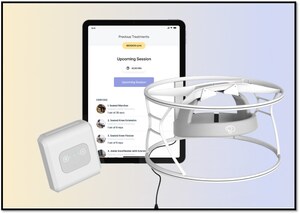
The BQ 2.0 system provides AI-powered, noninvasive, network-targeting electromagnetic precision therapy aimed at accelerating and enhancing the brain's recovery from stroke. Delivered via a cloud-based platform, BRAIN.Q's therapy is designed for seamless use by patients in both hospital and home settings.
"The past two decades have witnessed unprecedented advancements in our ability to efficiently train artificial intelligence learning systems. BRAIN.Q has harnessed these insights to design a precision therapy that helps the brain efficiently relearn and recover following a stroke. It's a big step toward our vision of restoring brain health and dignity of life for stroke survivors," said Yotam Drechsler, CEO and Co-founder of BRAIN.Q.
Stacey Pugh Appointed Chairman of the Board
In addition, the company announces the appointment of Stacey Pugh, as Chair of its Board. Ms. Pugh, a seasoned leader in the stroke space who led Medtronic's Neurovascular business, has been a dedicated member of BRAIN.Q's Board for the past three years.
"Stacey's leadership in the stroke space has been instrumental to our progress, and we are confident that under her chairmanship, BRAIN.Q will continue to push the boundaries of stroke care and bring meaningful solutions to patients worldwide," said Drechsler.
These milestones—the CE Mark approval and Ms. Pugh's appointment—represent major advancements in BRAIN.Q's journey to revolutionize stroke care. As the company continues to push forward, it remains dedicated to improving the lives of stroke survivors through breakthrough Restorative Brain Health therapeutics.
"I am so pleased to be stepping into this role at this time. Interventional stroke care has seen so many advances in the past decade, but still, over 60% of patients survive to live with a meaningful disability. BRAIN.Q's therapeutic approach allows us to further advance brain recovery and impact long-term outcomes for this group of people. Returning survivors to independence looks more and more possible, and I look forward to working with the company closely towards achieving this goal," said Pugh.
About BRAIN.Q
BRAIN.Q, a privately held US-Israeli startup, is dedicated to developing innovative, breakthrough therapies that aim to transform the lives of patients suffering from neurological disorders. The company has received Breakthrough Device Designation from the FDA for its Q Therapeutic System (formerly known as BQ) and was recognized as a groundbreaking innovation by the European Innovation Council.
Stay up to date on our key milestones, innovations, and the science behind our technology by visiting brainqtech.com, and following us on LinkedIn.
Legal Disclaimer:
Outside of the EEA, BRAIN.Q's therapy has not been approved and is limited to investigational use only. Please note that BRAIN.Q does not guarantee clinical trial outcomes or regulatory approval for its technology.
PRESS CONTACT:
Liat Reiffman Laufer
Digital Content Manager
P: +972-54-942-1773
E: [email protected]
SOURCE BrainQ





Share this article