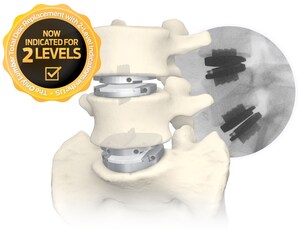
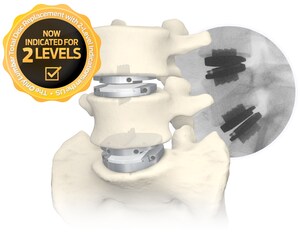
NEW YORK, April 2, 2019 /PRNewswire/ -- Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced that the North American Spine Society (NASS) has issued an updated coverage policy recommendation for lumbar artificial disc replacement. In the updated Coverage Policy Recommendation, NASS expanded its current position on clinical indications to include lumbar levels L3 to L4, matching the prodisc® L Lumbar Total Disc Replacement indications in its 2006 FDA PMA approval. prodisc L is the only FDA approved artificial disc replacement product indicated for use at lumbar levels L3 to S1.
The Position Statement goes one step further, by reiterating that reoperation rates at index levels in multiple meta-analyses were higher in fusion patients than in artificial disc replacement patients. Higher reoperation rates on fusion patients have a significant impact, including cost-effectiveness as well as quality-of-life metrics for patients. The Position Statement recommends coverage for lumbar artificial disc replacement when specific clinical indications are met.
"The NASS Statement reaffirms prodisc L indications, justifying the momentum of positive coverage policy decisions by carriers," says Centinel Spine Chairman & CEO, John Viscogliosi. "Over 30 million more patients have access to artificial disc replacement through their insurance carriers over the past year, explaining the up-swell in procedure volume and training inquiries." prodisc L was acquired by Centinel Spine one year ago, and has experienced significant procedure growth since the acquisition.
"This represents an excellent opportunity to provide my patients with a broader spectrum of treatment options," stated Jason Garber, MD, who practices in Las Vegas, Nevada, at the Las Vegas Neurosurgical Institute. "Having the North America Spine Society validate lumbar artificial disc replacement will ultimately enable more of my patients to have access to this beneficial treatment."
"The NASS coverage policy foretells a sweeping change in treatment paradigm for lumbar degenerative disc disease patients in the United States. In my current practice, I rarely fuse patients - there is now significant evidence that motion-preservation of the spine through artificial disc replacement is of the greatest long-term benefit," stated Jason M. Cuéllar, MD, PhD, Cedars-Sinai Medical Center, Los Angeles, CA.
Centinel Spine will highlight the Company's latest anterior column reconstruction technologies at the International Society for the Advancement of Spine Surgery (ISASS) Annual Meeting, held April 3-5, 2019 in Anaheim, California at the Anaheim Convention Center. At Booth #207, the company will showcase the prodisc Total Disc Replacement portfolio −the most extensive total disc replacement portfolio in the world- as well as its FLX™ 3D-Printed Porous-Titanium Interbody Devices. Additionally, Centinel Spine technologies will be highlighted in multiple podium presentations and a sponsored industry symposium.
About Centinel Spine, LLC
Centinel Spine®, LLC is the largest privately-held spine company focused on anterior column reconstruction. The company offers a continuum of trusted, brand-name motion-preserving and fusion solutions backed by over 30 years of clinical success — providing the most robust and clinically-proven Total Disc Replacement and Integrated Interbody™ portfolios in the world.
The company began operations in 2008 through the merger-acquisition of two pioneering medical device companies—Raymedica, LLC and Surgicraft, LTD. UK-based Surgicraft launched the first Stand-Alone/No Profile® anterior lumbar interbody fusion device in the world in 1988, which was the basis for future generations of the market-leading Integrated Interbody™ technology platform known today as STALIF®. Today, Centinel Spine still embraces the pioneering culture developed at both originating companies and continues its corporate mission to become the worldwide leading company addressing spinal disease anteriorly with the widest breadth & depth of technology platforms.
The company recently acquired the prodisc® Total Disc Replacement Technology Platform—the most extensive cervical and lumbar motion-preserving reconstruction portfolio available today. With the addition of prodisc, Centinel Spine stands alone as the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction.
Centinel Spine derived its name from the "Sentinel Sign", the radiographic confirmation of a successful fusion anterior to the interbody device.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
SVP, Corporate Finance & Strategic Planning
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
SOURCE Centinel Spine, LLC
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In



Share this article