Stryker Launches Revolutionary Computer Assisted Surgery Technology for Hip Fractures
KALAMAZOO, Mich., Oct. 4, 2012 /PRNewswire/ -- Stryker Corporation announced today that it has launched a revolutionary computer assisted surgery system, Stryker ADAPT™ for the Gamma3™ Locking Nail System.
Gamma3 nails are used to treat hip fractures, which are breaks in the upper end of the femur (the thighbone). There are approximately 300,000 hip fractures a year in the United States, and they are most commonly caused by falls or from direct impact to the side of the hip.1
The Gamma3 Locking Nail System consists of a cephalomedullary nail, a lag screw and a distal locking screw. The cephalomedullary nail is placed into the canal of the femur, and then the lag screw is placed through the nail and into the neck and head of the femur. The lag screw and nail together help unite the fracture, allowing it to become more stable to help promote proper healing.
Studies have shown that proper positioning of the lag screw in the femoral head is an important aspect of achieving positive patient outcomes. Failure of a cephalomedullary nail may occur if the lag screw has not been properly placed within the femoral head. A "cut out" of the lag screw in the femoral head is one potential result, which may necessitate a revision surgery.2
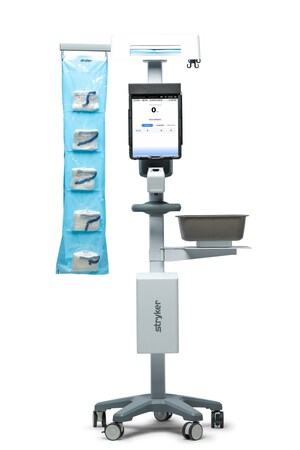
During conventional hip fracture surgery, surgeons use mechanical instruments and x-ray images to place the nail and lag screw. Stryker ADAPT is a computer assisted surgery system designed to assist surgeons in lag screw positioning by using Stryker's proprietary Adaptive Positioning Technology. The system automatically identifies the Gamma3 Locking Nail relative to the patient's anatomy and provides computer guidance to assist the surgeon with implant alignment, lag screw length and lag screw positioning.3
"We are pleased to offer the ADAPT/Gamma3 technology which assists surgeons to more accurately position the lag screw, with no significant difference in procedure time,"3 said Jim Bruty, Senior Director of Marketing, Stryker Navigation. "In addition, the number of x-ray images taken during the procedure can be reduced, which could potentially reduce the radiation exposure for the patient and the operating room staff."3
Stryker ADAPT for Gamma3 has been proven to assist surgeons in more accurately positioning the lag screw, regardless of their level of clinical experience.3 "Optimal lag screw placement has been identified as the primary factor in prevention of lag screw cut out," said Dr. James Maxey, orthopaedic surgeon in Peoria, IL. "The combination of clinical research, trauma engineering and elegant positioning technology allows novice and expert surgeons to accurately place the lag screw. The Stryker ADAPT system is a great innovation and advance in hip fracture repair."
"The Gamma System of implants and instruments has a 20-year history of continuous innovation to help surgeons achieve positive patient outcomes for hip fracture care," said Ken Gavlick, Senior Director of Hip Fracture Marketing, Stryker Trauma & Extremities.
Stryker ADAPT was designed specifically for use with Stryker's Gamma3 Locking Nail System and does not work with any other device. To learn more about Stryker ADAPT for Gamma3, please visit www.stryker.com/navigation.
- Wolters Kluwer Health UpToDate Website:Hip Fractures in Adults. Authors: Kevin E. Burroughs, MD and Katherine M. Walker, MD.
- The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. MR Baumgaertner, SL Curtin, DM Lindskog and JM Keggi. J. Bone Joint Surg. Am. 77:1058-1064, 1995.
- Internal Report. Stryker Osteosynthesis. Improved lag screw positioning in the treatment of proximal femur fractures using a novel computer assisted surgery method. January 2012. A series of cadaveric studies was conducted by Stryker to assess the impact of the ADAPT system on the lag screw placement during a Gamma3 surgery. In total, 45 surgeries were performed. The position of the lag screw within the femoral head and neck was assessed and analyzed using post-operative CT scans. The cadaveric studies were held at the Texas Health Research & Education Institute in Dallas (TX), USA on Sept. 9th and 10th, 2010 and at the Academy for Medical Training and Simulation, Luzern, Switzerland on April 4th and 5th, 2011.
About Stryker Corporation
Stryker is one of the world's leading medical technology companies and is dedicated to helping healthcare professionals perform their jobs more efficiently while enhancing patient care. The Company offers a diverse array of innovative medical technologies, including reconstructive, medical and surgical, and neurotechnology and spine products to help people lead more active and more satisfying lives. For more information about Stryker, please visit www.stryker.com.
MEDIA CONTACT:
Tamara Cutler
Vice President, Public Affairs
[email protected]
269-389-7253
| A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery. |
| The information presented is intended to demonstrate the breadth of Stryker product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area. |
SOURCE Stryker
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article