SAN DIEGO, Dec. 7, 2017 /PRNewswire/ -- Kerastem reports 6-month top-line data from its clinical study STYLE, a 70 patient, phase II, US multi-center, randomized, single-blinded, and controlled clinical trial investigating Kerastem's therapy in patients with early stage hair loss. The Kerastem therapy is a one-time treatment that utilizes adipose (fat) derived regenerative cells combined with purified fat delivered to the affected area of scalp.
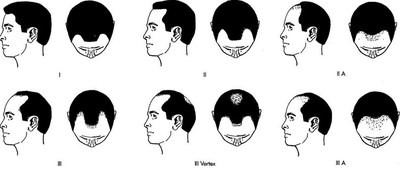
- Low dose Adipose Derived Regenerative Cells (ADRC's) + Puregraft fat treatment group achieved a statistically significant increase in mean terminal hair count, when compared to control, in males with early stage hair loss (Norwood Grades I-III). An average increase of 29 terminal hairs per cm2 of scalp was observed, corresponding to a 17% increase (p < 0.05) from baseline.
- All treatment arms of STYLE were safe with no serious adverse events reported.
"The Kerastem team has successfully identified a treatment that stimulates hair growth in people with early stage hair loss," stated Brad Conlan, CEO of Kerastem. "There are over 40 million people with early stage hair loss in the United States alone. Kerastem is poised to disrupt this market based upon the results from STYLE and is actively signing and exploring distribution relationships outside of the United States. We are thrilled about the STYLE data and what it means for patients with early stage hair loss who have had very few treatment options to date."
KERASTEM SUPERIOR TO OTHER CELL BASED TREATMENT CURRENTLY AVAILABLE
According to Dr. Eric Daniels, Kerastem's Chief Medical Officer, "STYLE indicates that dosing and tissue preparation are essential to achieving a successful outcome. Added Dr. Daniels, "We were very pleased to see the positive results from the low dose arm and were surprised that the high dose ADRC's + Puregraft fat treatment group, along with the fat alone group, observed a reduction, or no change, respectively in terminal hair counts. Stated otherwise, dosing and tissue purity matter."
To understand the STYLE results in context--the low dose treatment group had an average increase of 29 hairs per cm2 of scalp, which corresponds to an increase of 2,900 hairs per 100 cm2 of treated scalp. While the average hair transplant today is between 1,900 to 2,200 transplanted hairs, patients with early hair loss are very often not candidates for hair transplantation. Kerastem therapy represents a new option. The therapy utilizes patented medical device technology to deliver a consistent cell therapy output, enabling appropriate dosing.
ANDROGENETIC ALOPECIA MARKET SIZE
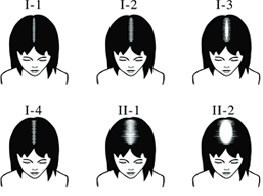
Hair loss affects more than 40 million men and 21 million women and in the United States alone. The global hair loss treatment market generates more than $7 Billion annually and currently has limited options for men (grades I-III) and women (grades I-II) with early stage hair loss.
ABOUT KERASTEM
Kerastem is the leader in the development and commercialization of cell-based approaches to hair growth. The company is a wholly owned subsidiary of Bimini Technologies. The Bimini portfolio also includes Puregraft, the world's leading fat transfer solution.
SOURCE Kerastem
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In

Share this article