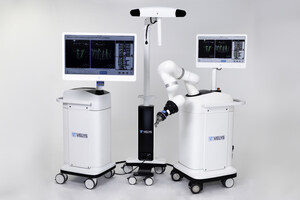
WARSAW, Ind., Aug. 7, 2017 /PRNewswire/ -- DePuy Synthes* has announced an exclusive agreement with Medical Enterprises Distribution, LLC to co-market the ME1000™ Surgical Impactor for use in Total Hip Arthroplasty (THA). The ME1000 is designed to replace the handheld mallet used in THA. The ME1000 delivers constant, stable energy that is designed to automate bone preparation, implant assembly and positioning. This in turn may lead to more consistent clinical outcomes and favorably impact patient satisfaction.
Because the battery-powered ME1000 automates THA, there is also likely to be less surgeon fatigue and potentially less work-related injuries that can arise from mallet use. A 2016 study published in The Journal of Arthroplasty showed that 66.1% of 183 orthopaedic surgeons who responded to a survey experienced a work-related injury and that 31% of responding surgeons required surgery for their injury.1 The paper cited the use of repetitive movements with operating instruments and tools as a potential cause for upper limb tendinitis and discussed the need for measures to improve the surgical environment and work ergonomics for orthopaedic surgeons.1
"In addition to its potential benefits for both patients and orthopaedic surgeons, the ME1000 can be easily integrated into a surgeon's operational technique and into the hospital's processes," said Aaron Villaruz, Global Hip Platform Leader, DePuy Synthes Joint Reconstruction. "We at DePuy Synthes view this technology as a significant advancement as we strive to help our customers meet their goals of improving clinical outcomes, increasing patient satisfaction and managing costs."
The ME1000 is compatible only with DePuy Synthes hip systems. Adapters are available for anterior and posterior approaches to THA. One of the early users of the technology is Dr. Joel Matta**, a pioneer in the Anterior Approach to Hip Replacement who is affiliated with the Steadman Clinic in Vail, CO.
"I've used the ME1000 on more than 100 primary DePuy Synthes hip implants, and my observation is that, compared to a mallet, the ME1000 reduces peak forces while increasing energy per second," said Dr. Matta. "I have found that the rotational stability of the implants is more easily and consistently achieved, and the risk of fracture potentially reduced. It is also easier for me to make adjustments, and achieve a precise position of the acetabular cup. I also appreciate that the physical effort and resultant fatigue from performing a hip replacement surgery is markedly reduced which benefits my capabilities during a full surgical schedule."
DePuy Synthes is expected to begin co-marketing the ME1000 with Medical Enterprises Distribution, LLC within the current quarter.
About DePuy Synthes Companies
DePuy Synthes Companies, part of the Johnson & Johnson Medical Devices Companies***, provides one of the most comprehensive orthopaedics portfolios in the world. DePuy Synthes Companies solutions, in specialties including joint reconstruction, trauma, craniomaxillofacial, spinal surgery and sports medicine, are designed to advance patient care while delivering clinical and economic value to health care systems worldwide. For more information, visit www.depuysynthes.com.
About Medical Enterprises Distribution, LLC
Based in Norcross, GA, Medical Enterprises Distribution, LLC, is a pioneering healthcare technology firm focused on surgical procedure innovation. For more information, visit http://www.medistribution.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995 regarding the effectiveness and value of the ME1000. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of DePuy Synthes and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; product efficacy or safety concerns resulting in product recalls or regulatory action; manufacturing difficulties and delays; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns for health care products and services; and trends toward health care cost containment. A further list and description of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended January 1, 2017, including under "Item 1A. Risk Factors", its most recently filed Quarterly Report on Form 10-Q, including in the section captioned "Cautionary Note Regarding Forward-Looking Statements", and the company's subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither DePuy Synthes nor Johnson & Johnson undertakes to update any forward-looking statement because of new information or future events or developments.
1 Alqahtani S, Alzahrani M, Tanzer M. Adult Reconstructive Surgery: A High-Risk Profession for Work-Related Injuries. The Journal of Arthroplasty 2016; 31(6): 1194-8.
*DePuy Synthes represents the products and services of DePuy Synthes, Inc. and its affiliates.
**Consultant to DePuy Synthes Joint Reconstruction with an ownership interest in Medical Enterprises Distribution, LLC.
***The Johnson & Johnson Medical Devices Companies comprise the surgery, orthopaedics, and cardiovascular businesses within Johnson & Johnson's Medical Devices segment.
©DePuy Synthes 2017. All rights reserved.
The third-party trademarks used herein are the trademarks of their respective owners.
SOURCE DePuy Synthes
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article